Document 13309653
advertisement

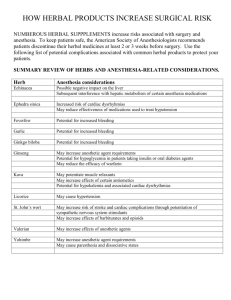
Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 01, Pages: 1-6 ISSN 0976 – 044X Research Article Simple Scheme for the Standardization of Herbal Drug: Swasanandam Gutika Deepa.P*, Babu. G, Mohammed Noufal.K, Mumthas .M, Shanthiya.K., Sreejisha.V.K. Devaki Amma Memorial College of Pharmacy, Chelembra, Kerala, India. *Corresponding author’s E-mail: deepa_kuttie@yahoo.com Accepted on: 24-04-2013; Finalized on: 31-03-2014. ABSTRACT The present study was undertaken to standardize the marketed formulation using simple, non-expensive methods. The standardization gives total information and controls to essentially guarantee consistent composition of herbal drugs. The present standardization involves pharmaceutical evaluation, organoleptic evaluation, and physical evaluation along with the quantitative estimation of alkaloids, flavonoids, saponins, total phenolics etc. Quality assurance is an integral part of traditional medicine, which ensures that it delivers the required quantity of quality medicament. This standardization helps quality control to assess safety, efficacy and quality of herbal medicine. Keywords: Extractive value, Ash value, total phenolic, flavonoid, saponins. INTRODUCTION T he W.H.O. has been encouraging and promoting the herbal medicines in health care programmes. Hence the standardization of the raw drugs, processing finished products, verification of the claims, mechanism of action and purity from metallic and microbial contamination were some of the major issue which have to be taken in to consideration for increasing the world wide acceptability of herbal products and also to achieve clinical success and maximum therapeutic effect1. According to an estimate of the world Health Organization (WHO), about 80% of the world population still uses herbs and other traditional medicines for their primary health care needs. Herbal formulations have reached widespread acceptability as therapeutic agents for diabetics, arthritics, liver disease, and cough remedies2. As per WHO definition, there are three kinds of herbal medicines: raw plant material, processed plant material and medicinal. Herbal formulations have reached extensive acceptability 3 as therapeutic agents for several diseases .The development of authentic analytical method which can reliably profile the phytochemical composition, including quantitative analyses of marker/ bioactive compounds and other major constituents, is a major challenge to scientists. Standardization is an important step for the establishment of a constituent biological activity, a consistent chemical profile, or simply a quality assurance programme for production and manufacturing of herbal drugs4. WHO mentioned specific guidelines for the assessment of safety, efficacy and quality of herbal medicines as a pre requisite for global harmonization were of utmost important 5. Standardization is a system that ensures a predefined amount of quality and therapeutic effect of ingredients in each dose6. Therapeutic activity of an herbal formulation depends on its phytochemical constituents. The development of authentic analytical methods reliably helps to profile the phytochemical composition, including quantitative analyses of marker or bioactive compounds. Standardization is an important step for the establishment of a consistent biological activity, a consistent chemical profile or simply a quantity assurance program for production and manufacturing of an herbal drug7. The authentication of herbal drugs and identification of adulterants from genuine medicinal herbs are essential for both pharmaceutical companies as well as public health and to ensure reproducible quality of herbal medicine. The subject of herbal drug standardization is massively wide and deep. For the purpose of research work on standardization of herbal formulations and nutraceuticals, a profound knowledge of the important herbs found in India and widely used in ayurvedic formulation is of utmost importance8. India needs to explore the medicinally important plants. This can be achieved only if the herbal products are evaluated and analyzed using sophisticated modern techniques of standardization. The world health organization (WHO) has appreciated importance of medicinal plant for public healthcare in a developing nations and has evolved guidelines to support the member states in their efforts to formulate national policies on traditional medicine and to study their potential usefulness including evaluation, safety and efficacy. Standardization of herbal drugs comprises total information’s and controls to essentially guarantee consistent composition of all herbals including analytical operations for identifications, markers, assay of active principles, there is no legal control model over medicinal plants9. Different counties medicinal plants or products derived from them in different ways and adopted different approaches of licensing, dispensing, International Journal of Pharmaceutical Sciences Review and Research Available online at www.globalresearchonline.net 1 Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 01, Pages: 1-6 manufacturing and trading to ensure their safety, quality and efficacy10. Swasanandam gutika belongs to the category bronchodilator. Chemical constituents in the formulation consists of Shudha hingula (purified and processed cinnabar), Karpoora (Cinnamomum camphora), Vatsanabha (purified and processed aconitum terox), Vara- tripala (Terminalia bellirica and Embilica officinalis). These medicines should only be taken under strict medical supervision. Self medication with this medicine may prove to be dangerous. It is to be avoiding in children and pregnant ladies, over dosage may cause many side effects including gastritis. ISSN 0976 – 044X between platens, one of which moves to apply deferent force to the tablet to cause fracture. The term crushing strength is also frequently used to describe the resistance of tablets to the application of a compressive load, and the result limit is ± 5%. Procedure The apparatus consist of two jaws facing each other, one of which more towards the other. Measurement was carried out on 10 tablets, taking care to remove all the fragments of the broken tablets before each determination and these takes average hardness. Limits-normal tablet hardness ranges from 3-6kg. MATERIALS AND METHODS 2. b. Friability Collection of materials It is the phenomenon where the surface of the tablet is damage shown site of damage due to mechanical shock. Marketed ayurvedic product was “Mangalodhayam Pharmaceuticals” Malappuram. collected from Changarakulam, Materials used Test Tube, Conical flask, Beaker, Muffle Furnace, Crucible, Petri dish, Funnel, Filter paper, Standard flask, Monsanto hardness tester, Roche friabilator, Hot air oven, U.V. Spectrophotometer, TLC plate, U.V. Chamber. The entire tests were carried out in the laboratory of Devaki Amma Memorial College of Pharmacy. Gutika was taken for standardization. Organoleptic characters and Physico chemical properties like colour, odour, taste, texture, pH, loss on drying, total ash, acid insoluble ash, water soluble ash, different extractive values etc were determined. Swasanandam gutika was studied for uniformity of weight and disintegration time. 1. Organoleptic evaluation Organoleptic characters of the sample were carried out based on color, shape, texture, odor, taste, pH, uniformity of weight, average weight of gutika were evaluated (Table 1). 1. a. pH pH of the formulations in 10% w/v of water soluble portions was determined using standard glass electrode at 24°C according to the prescribed standard method in Indian Pharmacopoeia. 1. b. Uniformity of weight 11 Procedure Accurately weighed the tablets, removed any loose dust from the tablet as before. A maximum mean weight loss from the 3 sample of not more than 1% was considered acceptable for most products. Percentage of friability of the tablets of a badge can be found by the following formula. Percentage friability = (W2/W1)100 W1-weight of tablets before testing W2-weightof tablets after testing 2. c. Disintegration tests11 Disintegration test was performed in a basket rack assembly supporting six glass tubes of 3 inches long, open at the top and held against a 10-mesh screen at the bottom end of the basket rack assembly. One tablet was placed in each tube and the assembly was moved up and down at a frequency of 30 cycles per min at a temperature of 37°C. 2. d. Fluorescence analysis: For fluorescence test, 1 mg of powdered drugs was exposed to ultraviolet light at wavelength of 365 nm and daylight while wet, after being treated with different reagents (Table 2). 3. Physico-Chemical evaluation Extractive values 20 Gutika of Swasanadham gutika was weighed individually and their average weight was calculated. 2. Physical Evaluation 2. a. Hardness test 11 Hardness is also so called crushing strength. It is the load required to crush the tablet when placed on its edge. Measure of the mechanical integrity of tablet is their breaking force, which the force required to cause them to fail in a specific plane. The tablets are generally placed This method determines the amount of active constituents in a given amount of marketed formulation when extracted with solvents. It is employed for that material for which no chemical or biological assay method exists. As mentioned in different official books the determination of water soluble and alcohol soluble extractives, is used as a means of evaluating crude drugs which are not readily estimated by other means. The extraction of any drug with particular solvents yields a solution containing different phyto constituents. The International Journal of Pharmaceutical Sciences Review and Research Available online at www.globalresearchonline.net 2 Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 01, Pages: 1-6 composition of these phytoconstituents in those particular solvents depends upon the nature of the drug and solvent used. The use of a single solvent can be the means of providing preliminary information on the quality of a particular drug sample. Procedure 3. a. Chloroform soluble extractive11 5gm of the air dried powder of the marketed sample was macerated with 100 ml chloroform in a stopper flask, and st was shaken frequently during the 1 6hrs and was allowed to stand for 18hrs. There after it was filtered rapidly taking precaution against the loss of solvent. 25ml of the filtrate was evaporated to dryness in a tarred flat bottom shallow disk dried at 105oC, and weighed and the percentage chloroform soluble extractive was calculated (Table 3). 3. b. Ethanol soluble extractive11 5 gm of the air dried powder of the given marketed sample was macerated with 100 ml ethanol in a stopper flask and was shaken frequently during the 1st 6 hrs and allow standing for 18hrs. There after it was filtered rapidly taking precaution against the loss of solvent. 25 ml of the filtrate was evaporated to dryness in a tarred flat bottom shallow disk dried at 105ᵒC, and weighed and the percentage of ethanol soluble extractive was calculated. 3. c. Water soluble extractive 11 5gm of the air dried powder of the given marketed sample was added in to 50 ml boiled water at 800c in a stopper flask. It was allowed to cool and filtered. 5ml of filtrate was transported to a tarred evaporating dish, other solvent was evaporated on a water bath and allowed to dry for 30min. Finally dried in an oven for 2 hrs and residue was weighed and the percentage of water soluble extractive was calculated (Table 3). 3. d. Ash values The ash of any material is composed of their non volatile inorganic components. Controlled incineration of marketed drug results in an ash residue consisting of an inorganic material. This value varies with in fairly wide limits and is there for an important parameter for the purpose of evaluation of the formulation. In certain formulation, the percentage variation of the weight of ash from sample to sample is very small and any marked difference indicates a change in quality. Unwanted parts of drugs, posses a character that will raise the ash value. More direct contamination such as by sand or earth is immediately detected by the ash value. The ash value can be determined by 3 different methods to measure the total ash, acid insoluble ash, water soluble ash (Table 3). Procedure 3. d. 1. Total ash11 Weighed accurately a quantity of the powdered ayurvedic tablet equivalent to 1 gm in a tarred platinum dish and ISSN 0976 – 044X o incinerated to a temperature not exceeding 550 C until free from carbon, approximately for about 4 hrs, cooled and weighed. The percentage of ash was calculated. 7,10 3. d. 2. Sulphated ash Silica or platinum crucible was made to redness for 10mins and allowed to cool in a desiccator and weighed. One gram of the substance accurately weighed was put in to crucible and ignited gently at first. Cooled, moistened the residue with 0.5ml of sulphuric acid, heated at 550oC for about 4hrs. Again cooled and weighed. Percentage of ash was calculated. 3. d. 3. Acid insoluble ash7,10 Powdered ayurvedic tablet equivalent to 1gm of the air dried drug was accurately weighed in a tarred platinum dish and incinerated to a temperature not exceeding o 550 C until free from carbon, approximately for about 4hrs, cooled and weighed. The percentage of ash was calculated. The total ash obtained by the above method was boiled for 5mints with 25ml of 2M hydrochloric acid and filtered through an ash less filter paper. The filter paper was ignited in the silica crucible, cooled and then acid insoluble was weighed. 4. Pharmacognostical evaluation Phytochemical screening was carried out in order to find the various constituents present in the herbal drug. The drug in methanol was tested for the presence of alkaloids, glycosides, steroids, flavonoids, tannins, phenolic compounds, proteins and carbohydrates.Table:4. 4. a. Heavy metals Limit tests were performed as per WHO prescribed procedure. 4. b. Presence of foreign organic matter Herbal drugs should be made from the stated part of the plant and be devoid of other plants of the some plants or the other plants. They should be entirely free from moulds or insect, including excreta and visible contaminant such as sand and stones, poisonous and harmful foreign matter and chemical residues. Animal matters such as insects and invisible microbial contaminants, which can produce toxins, are also among the potential contaminants of herbal medicines. Macroscopic examination can easily employ to determine the presence of foreign matter, although microscopy is indispensable in certain special cases. Procedure 100 grams of sample was weighed and spreaded the sample on a white tile or a glass plate uniformly without overlapping. The sample was inspected with naked eyes or by means of lens (5x or above). Foreign organic matter was separated manually. After complete separation, the matter was weighed and %weight by weight present in the sample was determined. International Journal of Pharmaceutical Sciences Review and Research Available online at www.globalresearchonline.net 3 Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 01, Pages: 1-6 4. c. Moisture content The determination of moisture in a substance is done for many purposes such as assessment of quality, quality control, quality assurance, detection and estimation of adulteration. The reproducibility results can be obtained under a set of standardized condition .The activity of any product depends on 1) Chemical composition of substance 2) The state of aggregation of its constituents 3) Water content Procedure11 1) The substance was placed and its weight was taken before measuring the moisture content. 2) The oven was heated to 200 degree for 3 hrs. 3) The substance was removed and weighed. 4) The drying of the substance was repeated at 200 degree in the interval and the substance was weighed between times until the last two weights remaining same indicating that the substance had reached constant mass. 5) The constant mass amount was subtracted from the weight before drying to determine the weight of moisture evaporated from the substance. 6) The weight of moisture in the substance was divided by the weight before drying and multiplied by 100 to determine the % of the substance. 4. d. TLC finger printing profile Preparation of extract of Swasanandam Gutika Refluxed 1gm of powdered drug with 25 ml methanol for 2hrs and filtered. Extracted further with another 25ml methanol for 1hr and again filtered. Evaporated the combined filtrate to dryness. Dissolved the residue in 25ml Methanol. TLC study of methanolic extracts of the individual ingredients, in Swasanandam Gutika (marketed formulation) were carried out along with the different marker compounds corresponding to the active ingredients. It ensured the presence of active ingredients . in the formulations ISSN 0976 – 044X using a capillary tube by keeping 1-3cm from the bottom of the TLC plate. The portion of the sample spots were marked. When the spot has been dried, the plate was placed in the chamber at angle of 45 degree. Here ascending development technique was used. It is important in TLC. Due to that the development chamber was perfectly saturated with the mobile phase. The solvent raised by capillary action dissolved the sample mixture into discrete spot. After that plate was removed from the tank. Thus solvent was marked and plate was dried The separated spots were identified by various physical methods, such as UV light and chemical method such as suitable reagent known as visualizing or spraying reagent. After the location of separated compounds, the next job is their identification. This was done by calculating Rf values of different compounds. Rf value = Distance travelled by solute from baseline Distance travelled by solvent Procedure The plate used was having 2cm width and 12cm in length. 1) Preparing of slurry 100gm of silica gel was weighed and mixed with 90 ml of water to produce slurry. The prepared slurry was poured on plate and spreaded all over the area of the plate. Dried and kept it in a hot air oven for 10 minutes. 2) Development and detection The plate was placed in mobile phase The mobile phase was Glacial acetic acid: Ethyl acetate: Diethyl ether in the ratio of 7:2:1. When the mobile phase came to a particular length 1cm below top, removed and passed iodine vapor. Then the plate was taken and measured the distance travelled by the solved front and solute by scale and the Rf value was calculated. Rf values of sample and markers were compared. 5. Quantification of ingredients by simple spectral analytical technique 5. a. Total phenolic content Thin layer chromatography is usually abbreviated as TLC. It is a form of solid, liquid, adsorption chromatography in which the stationary phase is thin layer of fine adsorbent evenly spreaded over a surface of a glass plate and mobile phase is flowing organic liquid. The principle underlying is adsorption. Total phenolic content of the sample was measured as gallic acid equivalent. In TLC the preparation was carried on a glass or glass plate which was coated with thin uniform layer of inert adsorbent such as silica or alumina. The plates were activated by removing the liquid associated with the thin layer as completely as possible. This could be done by drying thin plate. This made the adsorbent layer active .The solution of sample and markers were applied by From the stock solution 10-50 microgram per ml were prepared Preparation of Standard solution: 1 gm of the gallic acid dissolved in 1000 ml of distilled water, which was the stock solution. Preparation of sample solution: Sample scratched from the TLC was mixed with 5 ml of FC reagent and allowed to stand at room temperature for 5 minutes and then added 4 ml of 1 M sodium bicarbonate International Journal of Pharmaceutical Sciences Review and Research Available online at www.globalresearchonline.net 4 Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 01, Pages: 1-6 ISSN 0976 – 044X to the above mixture. After 10 minutes the absorbance was measured at UV spectrophotometer. A calibration curve was plotted. Total phenolics were quantified by the calibration curve obtained by measuring the absorbance of known concentration of Gallic acid (Table 5). The ash value 2.07% (sulphate ash), 5.1% (total ash) respectively which indicate that the value lies within the range. Qualitative phytochemical studies proved the absence of carbohydrate, proteins, amino acids and glycosides. 5. b. Total flavonoid content With Dragendroff reagent the drug shows a dark blue color in the visible reagent. In presence of sodium nitrite and sulphuric acid Dragendroff followed an intense dark blue color and the color persists for more than 10 minutes. Total flavonoids were measured as quercetin equivalents. Preparation of Standard solution: 1 gm of the quercetin dissolved in 1000 ml of distilled water, which was the stock solution. From the stock solution 10-50 microgram per ml were prepared Preparation of sample solution: To the sample scratched from the TLC , 0.2 ml of 10 % Aluminum chloride reagent,0.2 ml of potassium acetate were added and absorbance was recorded at 430 nm against the blank. The amount of flavonoid was calculated with reference to quercetin from the calibration curve (Table 5). Thin layer chromatography Aconitine (marker) shows the Rf value of 0.72 complies with the Rf value 0.69 of the sample, 1,8 cineole shows the Rf value of 0.39 complies with the Rf value 0.41 of the sample, Gallic acid shows 0.65 which resembles to 0.70 of Swasanandham gutika. Table 1: Organopleptic Evaluation Sl. No Characteristics Color Brown Shape Round 5. c. Total alkaloid content Texture Hard Total alkaloids were measured as aconitine equivalents. Odor Pungent Preparation of Standard solution: Taste Bitter Uniformity of weight Complies as per I.P reference. Avg .w t 400 mg pH Acidic 1 gm of the aconitine was dissolved in 1000 ml of distilled water, which was the stock solution. From the stock solution 10-50 microgram per ml was prepared. Preparation of sample solution: Table 2: Flouresence analysis Reagents Results Sample in methanol was treated with2 ml of 0.5% dragendroff reagent and was allowed to stand for 10 minutes and the absorbance was read at 438 nm against the blank. The amount of alkaloid was calculated with reference to aconitine from the calibration curve (Table 5). With Dragendroff reagent in visible + Dragendroff reagent followed by sodium nitrite in visible + Dragendroff reagent +sulphuric acid in visible + 10 % Sulphuric acid in UV 365 nm - RESULTS AND DISCUSSION Table 3: Results of Physical, Physico-Chemical and pharmacognostical evaluation Tablets must be able to withstand the rigors of handling and transportation experienced in the manufacturing plant, in the drug distribution system and on the field at the hands of the end users (patient or consumers). The value of percentage friability was found to be 0.695%, which indicates that the value lies within the range. Purpose of friability is to evaluate the ability to the tablets to withstand the breakage during the transportation and handling. Characters Results Hardness test 3.5 kg/cm Friability 0.695 % Disintegration test complies with I.P Water soluble extractive 5.6% Chloroform soluble extractive 3.26 3 Methanol soluble extractive 2.84 Moisture present in the formulation leads to degradation process. The moisture content was found to be 0.22% Sulphated ash value 2.07 % Total ash 5.1% The extractive value were found to be 3.2 % (chloroform extract), 2.8 % (ethanol extract) and 5.6% (water soluble extract). Acid insoluble ash 4.85 Moisture content 0.22 %. % of foreign matter nil Heavy metals nil International Journal of Pharmaceutical Sciences Review and Research Available online at www.globalresearchonline.net 5 Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 01, Pages: 1-6 Table 4: Results for preliminary phytochemical studies Phyto constituents Report Carbohydrates - proteins - Amino acids - steroids + Triterpenoids + Glycosides - Flavonoids + Alkaloids + Tannins & phenolic compounds + Table: 5. Quantification of ingredients in the marketed sample. Parameters Inference Total Phenolics 12±7 mg/g Total Alkaloids 45±10 mg/g Total Flavonoid 32±2 mg/g ISSN 0976 – 044X REFERENCES 1. Sagar Bhanu PS, Zafar R, Herbal drug standardization, The Indian Pharmacist, 4(35), 2005,19 - 22. 2. Amit J, Sunil C, Phytosomes: A, revolution in herbal drugs, The Pharma Review, 2007, 11-13. 3. Patel PM, Patel NM, Goyal RK , Quality control of herbal products, The Indian Pharmacist, 5(45), 2006, 26-30. 4. Vaidya ADB, Devasagayam TPA, Current status of herbal drugs in India: An overview, J Clin Bio chem, 41(1), 2007, 1-11. 5. Yadav NP, Dixit VK, Recent approaches in herbal drug standardization, Int J Integr Biol, 2, 2008,195-203. 6. Patra KC, Pareta SK, Harwansh RK, Traditional approaches towards standardization of herbal medicines-A review, J Pharm Science Techno, 2 (11), 2010,372-379. 7. Indian Herbal Pharmacopoeia, Indian Drug Manufacturers Association, Mumbai, 2002. 8. British Herbal Pharmacopoeia, British Herbal Medicine Association, 1996. 9. Quality Control Methods for Medicinal Plant Materials, WHO, Geneva, 1996. 10. National Health Policy, Ministry of Health and family Welfare, Government of India, New Delhi 1983. CONCLUSION Physical, phytochemical and the pharmacognostic study have been useful for deciding the identity, purity and strength of the marketed formulation. The present study provides a brief report on herbal drug swasanadhi gutika. The standardization helps to build a monograph of the Ayurvedic formulation in the Indian Formulary. This study need to be continued. 11. Sharma AK, Gaurav SS, A rapid and simple scheme for the standardization of polyherbal Drugs , Int J Green pharm, 2009, 134-140. Source of Support: Nil, Conflict of Interest: None. International Journal of Pharmaceutical Sciences Review and Research Available online at www.globalresearchonline.net 6