CYP2C19*2 FOR PHARMACIST-LED PERSONALISATION OF ANTIPLATELET THERAPY
advertisement

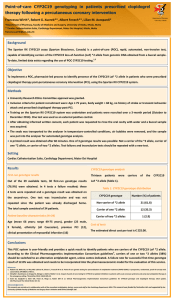
COMPARISON BETWEEN A POINT-OF-CARE AND A LABORATORY-BASED CYP2C19*2 GENOTYPING ASSAY FOR PHARMACIST-LED PERSONALISATION OF ANTIPLATELET THERAPY DEPARTMENT OF PHARM ACY UNIVERSI TY OF MA LTA Francesca Wirth*, Graziella Zahra**, Robert G. Xuereb***, Christopher Barbara**, Albert Fenech***, Lilian M. Azzopardi* *Department of Pharmacy, Faculty of Medicine and Surgery, University of Malta, Msida, Malta ** Molecular Diagnostics Unit, Department of Pathology, Mater Dei Hospital, Msida, Malta ***Cardiac Catheterisation Suite, Department of Cardiology, Mater Dei Hospital, Msida, Malta Department of Pharmacy University of Malta Email: francesca.wirth@um.edu.mt INTRODUCTION METHOD RESULTS The novel point-of-care (POC) Spartan™ RX After obtaining written informed consent, patients undergoing PCI with stent The patient cohort consisted of 34 patients. Twenty-five patients were male and 9 were female, mean age was 66 years (range 49-75 years) and all patients were Caucasian. has not been previously compared to deployment for acute coronary syndrome or stable angina and who were candidates for dual antiplatelet therapy, were recruited by non-probability sampling from the catheterisation laboratory at Mater Dei Hospital (MDH). Exclusion criteria were patients age < 18 years and > 75 years, body weight < 60 kg, history of stroke or transient ischaemic attack, active bleeding, coagulation or platelet disorders, and/or chronic liver disease. laboratory-based CYP2C19*2 genotyping A buccal swab and EDTA-whole blood sample were obtained for CYP2C19*2 CYP2C19 genotyping assay rapidly identifies presence of the CYP2C19 loss-offunction ‘*2’ variant allele and can be applied to personalise antiplatelet therapy at the start of treatment. 1-3 This POC assay using reverse dot-blot (RDB) hybridisation. AIMS To apply the Spartan™ RX assay for pharmacist-led CYP2C19*2 genotyping to personalise antiplatelet therapy in patients undergoing percutaneous coronary intervention (PCI) and compare it to a laboratory-based CYP2C19*2 genotyping assay which uses a robust and accurate RDB hybridisation technique. genotyping with the POC Spartan™ RX CYP2C19 assay (Spartan Bioscience) and with the laboratory-based GenID® RDB hybridisation 2070/1X assay (Autoimmun Diagnostika AID GmbH) respectively. Each patient was genotyped as a non-carrier of CYP2C19*2 (*1/*1), a carrier of one *2 allele (*1/*2), or a carrier of two *2 alleles (*2/*2). Genotypes requiring alternative antiplatelet therapy to clopidogrel (*1/*2, 4 *2/*2) were verbally communicated to the cardiologist and treatment decision was taken by the cardiologist. All processes were led by a clinical With the POC Spartan™ RX assay, 21 patients were genotyped as non-carriers of the CYP2C19 *2 allele, 12 patients as carriers of one *2 allele and 1 patient as a carrier of two *2 alleles. With the GenID® assay, the same 21 patients were genotyped as non-carriers of the CYP2C19 *2 allele, however 13 patients were genotyped as carriers of one *2 allele and no patients were identified as carriers of two *2 alleles. Agreement in genotype results was 97% Type of test system Polymerase chain reaction-based Sample type for genomic DNA Technique Approximate timefor-result (hours) User-friendliness of test procedure Sample batching Interpretation of results Assay storage Controls Estimated cost (€) per test (consumables only) Spartan™ RX assay Point-of-care Yes GenID® RDB assay Laboratory-based Yes with reverse hybridisation Buccal swab EDTA-whole blood Non-invasive Invasive 1 6 Simple procedure Laborious with no laborious procedure with preparation and extensive training very minimal required training required Not required Not required however may be more practical Very user-friendly Very user-friendly Strict requirement Fridge 2-8 °C and for manual defrost any type of freezer freezer at –20 °C at –20°C or below or below Internal Internal 225 23 Table 1: Qualitative comparison of assays used (κ=0.939). pharmacist researcher. Comparison between genotype results obtained with the two assays was carried out using percentage agreement and Cohen’s kappa (κ) statistic. CONCLUSION The POC Spartan™ RX assay is accurate and reliable (97% agreement in genotype results). The single mismatched result does not 4 impact personalisation of antiplatelet therapy since an alternative to clopidogrel is recommended for both carriers of one and two CYP2C19 LoF *2 alleles (13 patients in the cohort studied). The POC assay provides much faster results, requires minimal training to perform the test and is non-invasive, however the tests are more expensive. References 1. Roberts JD et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): A prospective, randomised, proof-of-concept trial. Lancet 2012; 379 (9827): 1705-11. 2. Stimpfle F et al. Impact of point-of-care testing for CYP2C19 on platelet inhibition in patients with acute coronary syndrome and early dual antiplatelet therapy in the emergency setting. Thromb Res 2014; 134(1):105-10. 3. So DY al. A prospective randomized evaluation of a pharmacogenomic approach to antiplatelet therapy among patients with ST-elevation myocardial infarction: The RAPID STEMI study. Pharmacogenomics J 2015 [Epub ahead of print]. 4. Scott et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther 2013; 94 (3): 317-23. Acknowledgements This research was carried out in collaboration with all the consultant cardiologists, doctors, nurses and staff at the Department of Cardiology, MDH. Financial Support: University of Malta Faculty of Medicine and Surgery Dean’s Initiative, Technoline Ltd., Scientech Ltd., Malta Heart Foundation, Autoimmun Diagnostika AID GmbH, Orme Scientific Ltd., LEVO Laboratory Services Ltd.