VITAMIN D, 25 HYDROXY 12/9/2014 LABP000102
advertisement
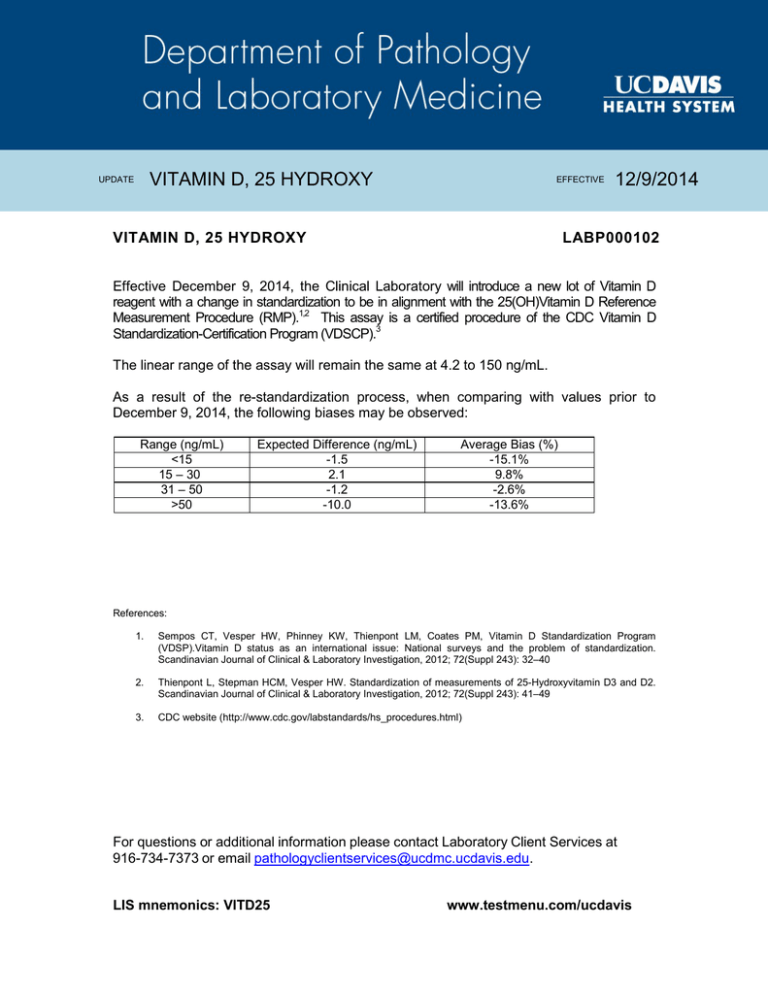
VITAMIN D, 25 HYDROXY UPDATE EFFECTIVE VITAMIN D, 25 HYDROXY 12/9/2014 LABP000102 Effective December 9, 2014, the Clinical Laboratory will introduce a new lot of Vitamin D reagent with a change in standardization to be in alignment with the 25(OH)Vitamin D Reference Measurement Procedure (RMP).1,2 This assay is a certified procedure of the CDC Vitamin D Standardization-Certification Program (VDSCP).3 The linear range of the assay will remain the same at 4.2 to 150 ng/mL. As a result of the re-standardization process, when comparing with values prior to December 9, 2014, the following biases may be observed: Range (ng/mL) <15 15 – 30 31 – 50 >50 Expected Difference (ng/mL) -1.5 2.1 -1.2 -10.0 Average Bias (%) -15.1% 9.8% -2.6% -13.6% References: 1. Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, Vitamin D Standardization Program (VDSP).Vitamin D status as an international issue: National surveys and the problem of standardization. Scandinavian Journal of Clinical & Laboratory Investigation, 2012; 72(Suppl 243): 32–40 2. Thienpont L, Stepman HCM, Vesper HW. Standardization of measurements of 25-Hydroxyvitamin D3 and D2. Scandinavian Journal of Clinical & Laboratory Investigation, 2012; 72(Suppl 243): 41–49 3. CDC website (http://www.cdc.gov/labstandards/hs_procedures.html) For questions or additional information please contact Laboratory Client Services at 916-734-7373 or email pathologyclientservices@ucdmc.ucdavis.edu. LIS mnemonics: VITD25 www.testmenu.com/ucdavis








