TOPO II Zero Blunt PCR Cloning Kit
advertisement

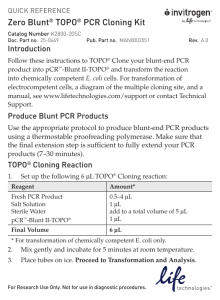
TOPO II Zero Blunt PCR Cloning Kit [Notes: For blunt-end cloning (NO TA-overhang, don’t use Taq-based) of PCR fragments. Kit recommends not exceeding 3,000 bp inserts (and don’t use fragments that are too small either). Use high fidelity Polymerase (e.g. KOD hot start or other)] 1. Confirm correct size PCR product on gel (multiple bands or mispriming—redo PCR conditions). 2. Mix TOPO II reaction at room temperature (swirl with tip, no vortex) 4.5 uL water 1.0 uL salt solution (included) 1.0 uL TOPO II 0.5-1.0 uL of PCR reaction 3. Incubate at 25°C for 10-15 minutes in a rack. 4. Once reaction is incubating, get tube(s) of competent E. coli. Can use either the purplecapped Invitrogen proprietary cells (included with the kit—50uL cells), or use aliquot of the lab’s TOP10 competent coli (can use 50-100 uL) and incubate on ice for 10 minutes. 5. Add 2 uL of the TOPO reaction to the competent coli. Do NOT pipet up and down. Mix very gently with tip and place on ice for 5-10 additional minutes. 6. Heat shock at 42°C for 30 seconds. 7. Place on ice for 2 minutes. 8. Add 250 uL SOC media (or 2xLB works fine too) and place on rotator at 37°C for 1 hour. 9. Plate full volume onto LB+Kan plate (TOPO II vector is KanR). 10. Incubate at 37°C overnight. 11. Miniprep up single colonies (1-4 colonies) and test first by restriction digest (recommended Not1HF/Spe1HF double digest, or other if your insert HAS one of these sites) to confirm the addition of your PCR fragment into the TOPO II vector. 12. For a SINGLE correct (via digest) isolate, send to sequencing with M13F and/or M13R sequencing primers (included with kit) depending on the size of your insert. This confirms that the inserted PCR fragment is correct (sequence) and confirms the orientation (which direction it was inserted into the vector since the blunt-end cloning is bi-directional). 13. Save correct isolate into Vector Database.