Fundamental Properties of an Oxygen-Damaged Small
advertisement

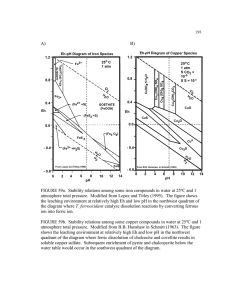
Fundamental Properties of an Oxygen-Damaged Small Molecule Model of Fe-only Hydrogenase: Studies in Intra- and Intermolecular Ligand Exchange Bin Li (Ben) Group of Dr. Marcetta Y. Darensbourg Department of Chemistry, TAMU An Oxygen-Damaged Small Molecule Model of Fe-only Hydrogenase OC e- H2 X Cys Fe Fe NC (b) Fe C O CO C N C O X= CH2, NH or O (a) Fe CO C O S S [4Fe4S] S H+ OC C O O C SS O OC OC C O SS Fe O C Fe CO C O (c) Active site of [FeFe] H2ase (a); Model Complex (b); Oxygen-Damaged Model Complex (c). What is the influences of Sulfenato-oxygen upon the ligand exchange into such oxygen damaged models? Previous Kinetic Studies of Model of [FeFe]H2ase Active Sites Marcetta Y. Darensbourg’s group Thomas B. Rauchfuss’ and Luca De Gioia‘s group Rate = k2[FeFe][CN-] Eric J, Lyon, Marcetta Y. Darensbourg et al, J.Am.Chem.Soc. 2001, 123, 3268-3278. Aaron K. Justice, Luca De Gioia, Thomas B. Rauchfuss et al. Inorg. Chem. 2007,46,1655-1664. Kinetic Studies of PMe3/CO Exchange into (μ-pdt)Fe2(CO)6 OC OC O C SS Fe C O Fe OC PMe3 Fe OC Me3P CO C O (μ-pdt)Fe2(CO)6 + xs PMe3 SS Toluene (μ-pdt)Fe2(CO)5PMe3+ xs PMe3 Rate = k2[(μ-pdt)Fe2(CO)6][PMe3] Fe O C PMe3 CO C O OC Fe OC Me3P (μ-pdt)Fe2(CO)5PMe3 Toluene O C SS Fe PMe3 C O (1) (μ-pdt)Fe2(CO)4(PMe3)2 (2) Rate = k2’[(μ-pdt)Fe2(CO)5PMe3][PMe3] Determination of Rate Constants 3 2.5 ln(A0/At) 2 ln(At/A0)= kobst 1.5 1 0.5 0 0 20 40 60 80 100 120 Time, (min) Figure 4. Example of plot of ln(A0/At) vs time over five half-lives, Entry 4, Table 1. -7.6 -7.8 ln(kobs ) -8 kobs=k2[PMe3] -8.2 -8.4 y = 1.03x - 6.48 R2 = 0.997 -8.6 -8.8 -9 -2.4 -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 ln[PMe3] Figure 5. Plot of ln(kobs) vs ln[PMe3] for the formation of (μ-pdt)Fe2(CO)5PMe3 from (μ-pdt)Fe2(CO)6 measured at 22°C. Rate Constants of PMe3/CO Exchange into (μ-pdt)Fe2(CO)6 Table 3. Rate Data for the Reaction of PMe3 with (μ-pdt)Fe2(CO)6 (1) Measured at 22 °C and with (μ-pdt)Fe2(CO)5PMe3 (2) Measured at 50 °C in Toluene Solution entry compd [PMe3], M 104 kobs, sec-1 1 1a 0.10 1.47 2 1 0.15 2.15 3 1 0.20 2.85 4 1 0.30 4.55 av k2 = 14.6 (± 0.6) × 10-4 M-1 sec-1 5 2a 0.10 1.28 6 2 0.15 1.97 7 2 0.20 2.62 8 2 0.30 4.00 av k2 = 13.1 (± 0.3) × 10-4 M-1 sec-1 a [Fe 2] = 5.0 × 10-3 M with 20 — 60-fold excess PMe3. Kinetic Studies of PMe3/CO Exchange into (μ-pst)Fe2(CO)6 O OC OC SS Fe C O O C Fe PMe3 CO C O (μ-pst)Fe2(CO)6 + xs PMe3 O OC SS Fe OC Me3P Toluene O C Fe CO C O (μ-pst)Fe2(CO)5PMe3 Rate = k2[(μ-pst)Fe2(CO)6][PMe3] Determination of Rate Constants Table 4. Rate Data for the Reaction of PMe3 with (μ-pst)Fe2(CO)6 (1) Measured at 95 °C in Toluene Solution a [Fe 2] entry compd [PMe3], M 104 kobs, sec-1 1 3a 0.10 0.43 2 3 0.20 0.62 3 3 0.30 0.85 4 3 0.40 1.07 = 5.0 × 10-3 M with 20 — 80-fold excess PMe3. -9 Rate = k2[(μ-pst)Fe2(CO)6][PMe3] -9.2 ln(kobs ) -9.4 kobs=k2[PMe3] y = 0.65x - 8.59 R2 = 0.986 The reaction does not follow a second-order rate expression well. It suggested a complicated, both SN2 and SN1 substituted process was involved at a high reaction temperature with dissociation of CO ligand of (μ-pst)Fe2(CO)6. -9.6 -9.8 -10 -10.2 -2.7 -2.2 -1.7 -1.2 -0.7 ln[PMe3] Figure 8. Plot of ln(kobs) vs ln[PMe3] for the formation of (μ-pst)Fe2(CO)5PMe3 from (μ-pst)Fe2(CO)6 measured at 95°C. Kinetic Studies of CN-/CO Exchange into (μ-pst)Fe2(CO)6 1OC OC SS Fe C O O OC O C Et4N+CN- Fe CO OC C O (μ-pst)Fe2(CO)6 + xs Et4NCN Fe O C Et4N+CN- Fe CO C N C O CH3CN (μ-pst)Fe2(CO)5(CN)- + xs Et4NCN Rate = k2[(μ-pst)Fe2(CO)6][CN-] O SS CH3CN 2OC OC SS Fe C N O O C Fe CN C O (μ-pst)Fe2(CO)5(CN)(μ-pst)Fe2(CO)4(CN)22- Rate = k2’[(μ-pst)Fe2(CO)5(CN)-][CN-] Determination of Rate Constants 1.4 -8.2 1.2 -8.4 -8.6 ln(kobs) ln(A0/At) 1 0.8 -8.8 -9 0.6 y = 1.00x - 7.14 R2 = 0.982 -9.2 0.4 -9.4 0.2 -9.6 -2.4 0 0 10 20 30 40 50 60 70 -2.2 80 -2 -1.8 -1.6 -1.4 -1.2 ln[CN ] Time, (min) Figure 11. Plot of ln(kobs) vs ln[CN-] for the formation of (μ-pst)Fe2(CO)4(CN)22from (μ-pst)Fe2(CO)5(CN)- measured at 40°C. Figure 10. Example of plot of ln(A0/At) vs time over three half-lives. Table 5. Rate Data for the Reaction of Et4N+CN- with (μ-pst)Fe2(CO)6 (3) Measured at 0 °C and with [Et4N+ ][(μ-pst)Fe2(CO)5(CN)-] (4) Measured at 40 °C in CH3CN Solution a [Fe b [Fe entry compd [CN-], M 104 kobs, sec-1 1 3a 0.10 22.43 2 4b 0.10 0.83 3 4 0.15 1.08 4 4 0.20 1.62 5 4 0.30 2.43 = 5.0 × 10-3 M with 20-fold excess CN-. -3 2] = 5.0 × 10 M with 20 — 60-fold excess CN . 2] -1 - av k2 = 7.94 (± 0.8) × 10-4 M-1 sec-1 Determination of Activation Parameters Eyring plot Arrhenius plot -4 -10 -10.5 -5 -11.5 ln k2 ln(k2/T) -11 -12 -12.5 -6 -7 -13 -13.5 -8 2.8 2.9 3 3.1 3.2 3.3 3.4 3.5 3 1/T (×10 ) Figure 12. Eyring plot for the formation of (μ-pdt)Fe2(CO)5PMe3 from (μpdt)Fe2(CO)6 (□), formation of (μ-pdt)Fe2(CO)4(PMe3)2 from (μpdt)Fe2(CO)5PMe3 (△) and formation of (μ-pst)Fe2(CO)4(CN)22- from (μpst)Fe2(CO)5(CN)- (○). 2.8 2.9 3 3.1 3.2 3.3 3.4 3.5 1/T (×103) Figure 13. Arrhenius plot for the formation of (μ-pdt)Fe2(CO)5PMe3 from (μ-pdt)Fe2(CO)6 (□), formation of (μ-pdt)Fe2(CO)4(PMe3)2 from (μ-pdt)Fe2(CO)5PMe3 (△) and formation of (μ-pst)Fe2(CO)4(CN)22from (μ-pst)Fe2(CO)5(CN)- (○). ln(k2/T) = (-ΔH‡/R)T-1 + ln(kB/h) + (ΔS‡/R) k2 = A exp(-Ea / RT) ln(k2/T) vs T-1 lnk2 vs T-1 Slope= -ΔH‡/R, Intercept = ln(kB/h) + (ΔS‡/R) Slope= -Ea/R Determination of Activation Parameters Table 6. Temperature Dependence of Reaction of PMe3 with (μ-pdt)Fe2(CO)6, (μ-pdt)Fe2(CO)5PMe3 and Reaction of Et4N+CN- with [Et4N+ ][(μ-pst)Fe2(CO)5(CN)-] compound T,°C (104) k2, M-1s-1 activation parameters 22 14.67 Ea = 48 kJ/mol 30 25.50 ΔH‡ = 45 kJ/mol 40 45.00 ΔS‡ = - 126 J/mol K 50 80.83 50 12.83 Ea = 53 kJ/mol 60 26.67 ΔH‡ = 50 kJ/mol 70 44.83 ΔS‡ = - 127 J/mol K 80 68.67 40 8.33 Ea = 61 kJ/mol 50 16.33 ΔH‡ = 58 kJ/mol 60 31.17 ΔS‡ = - 99 J/mol K 70 65.50 (μ-pdt)Fe2(CO)6a (μ-pdt)Fe2(CO)5PMe3a [Et4N+ ][(μ-pst)Fe2(CO)5(CN)-]b a [Fe2] = 5.0 × 10-3 M; [PMe3] = 0.1 M. b [Fe2] = 5.0 × 10-3 M; [CN-] = 0.1 M. Proposed mechanism for CN-/CO exchange reaction in (μ-pst)Fe2(CO)6 OC O SS Fe OC CN- O C Ea Fe SS Fe OC CO C O OC N C Fe C O C O C O O CO C O CO CN- OC O SS Fe OC - N C OC Fe Fe OC CO C O SS O Fe CO C O C O N C C O Ea' -NC OC SS Fe C O O N C Fe C O CO C O -- CO NC OC SS Fe C O O N C Fe CO C O We propose that the transition states play an critical role in the intermolecular PMe3/CO and CN-/CO exchange processes. The sulfenato-oxygen influence the coordination sphere intramolecular rearrangements due to steric and electronic effects. The increase of rotation barriers due to the presence of sulfenato-oxygen interaction thus increases the activation energy for the CN-/PMe3 attack. Conclusions • The PMe3/CO exchange process into (μ-pdt)Fe2(CO)6 follows a strict second-order rate law which is first order in both the substrate and PMe3. The evolved results suggest an associative or Ia mechanism. • The larger activation energy barrier of PMe3/CO exchange process into (μpst)Fe2(CO)6 responds to the high reaction temperature, which finds both SN2 and SN1 substituted mechanism occurred. • CN- exchanges with CO into (μ-pst)Fe2(CO)6 by associative processes produce both stable monocyano anionic and dicyano dianionic products. The second step CN-/CO exchange reaction was found to be rate-limiting step, which is converse to the CN-/CO exchange process into (μ-pdt)Fe2(CO)6.