Supplemental Checklist for Investigators and Staff Proposal Submission
advertisement

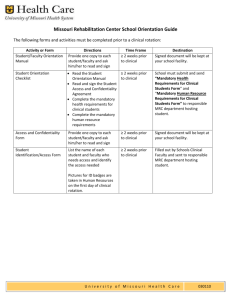
Supplemental Checklist for Investigators and Staff Proposal Submission (NIH, State, Foundations) Table of Contents Helpful Contacts ................................................................................................................. 3 Activity #1. Complete Necessary Training......................................................................... 5 Activity #2. Licenses and Approvals .................................................................................. 6 Activity #3. Grant Development ......................................................................................... 7 Activity #4. Prepare proposal Packet .................................................................................. 8 Activity #5. Submit to SOM Sponsored Programs ........................................................... 10 Activity #7. Granting Agency Activities .......................................................................... 12 Activity 8. Set up Accounts .............................................................................................. 13 Activity #9. Grant Maintenance ........................................................................................ 14 Activity #10. Grant Close out ........................................................................................... 15 Helpful Contacts Name Title Lars Berglund, M.D., Ph.D. Ted Wun, M.D. Senior Associate Dean for Research/Director of the UC Davis Clinical and Translational Research Center (CTSC) Associate Dean for Research Ted Wandzilak, Ph.D. Director, SOM Office of Sponsored Programs Gail DotsonSinclair Analyst, SOM Office of Sponsored Programs Lucy Moua Admin Asst, SOM Office of Sponsored Programs Kate Marusina, Ph.D., MBA Manager, Clinical Trials, CTSC Diane Hoffman SOM Safety Officer Jeffrey Elias, Ph.D. Director, SOM Office of Research Grant Coordination Unit Erica Chédin, Ph.D. SOM Office of Research, Grant Coordination Officer Area of Responsibility Responsible for all research activities within the SOM. Office of Research, Sponsored Programs, CTSC. Phone e-mail 916-7039120 Lars.berglund@ucdmc.ucdavis.edu Clinical Research within the SOM & CTSC. SOM Office of Research & Office of Sponsored Programs. Grant and Contract proposal review/SOM Grant database IRB, Grant & Contract review/SOM Grant database Unit provides support for clinical research billing, IRB preparation, budgets, clinicaltrials.gov and study monitoring Laboratory safety officer, ensuring compliance with Environmental Health and Safety policies Grants Writer, Assists with scientific proposal preparation & summary statement review. Focus on R01 type grants. Grants Writer, Assists with scientific proposal preparation. Focus on Center and Program Project Grant 916-7343771 Ted.wun@ucdmc.ucdavis.edu 916-7039138 Theodore.wandzilak@ucdmc.ucdavis.edu 916-7039139 Gail.dotson-sinclair@ucdmc.ucdavis.edu 916-7039141 Lucy.moua@ucdmc.ucdavis.edu 916-7039177 Kate.marusina@ucdmc.ucdavis.edu 530-7529996 Dianne.hoffman@ucdmc.ucdavis.edu 916-7039223 Jeffrey.elias@ucdmc.ucdavis.edu 916-7039145 Erica.chedin@ucdmc.ucdavis.edu Betty Guo, Ph.D. SOM Office of Research, Grant Coordination Officer William Ferrier, DVM Director, Large Animal Surgery Research Facility Teresa Farley MSO Center for Healthcare Policy and Research Proposals Grants Writer, Assists with scientific proposal preparation. Focus on Training Grant Proposals & Manuscripts Management of large animal surgery research ORU manager of a unit that provides full service proposal and award support 916-7039137 Betty.guo@ucdmc.ucdavis.edu 530-7549682 wtferrier@ucdavis.edu 916-7344665 tlfarley@ucdavis.edu Activity #1. Complete Necessary Training Learning objective: To prepare for grant submission Links NIH State Foundations Office of Sponsored Programs Submission Packet Course Contact Gail Dotson highly advised highly advised highly advised UCDHS Grants Preparation Course http://ucdmc.ucdavis.edu/ctsc/ar ea/core/core-facilitation.html Highly advised Highly advised Highly advised Conflict of Interest lms.ucdavis.edu (COIPHS-UCECO) Mandatory Mandatory Mandatory for some foundations Annual Lab Safety Training http://www.ucdmc.ucdavis.edu/ medresearch/medsp/labsafety.ht ml mandatory mandatory mandatory Animal Care and Use training (if needed for post-award) http://safetyservices.ucdavis.edu/ training/animal-care-and-usetraining Mandatory (if applicable) Mandatory (if applicable) Mandatory (if applicable) CITI Training http://www.research.ucdavis.edu /u/a/irb Mandatory (if applicable) Mandatory (if applicable) Mandatory (if applicable) Injury& Illness Prevention Program http://safetyservices.ucdavis.edu/ programs-and-services/injuryillness-prevention-iipp/injuryillness-prevention-program-iipp1 Mandatory mandatory mandatory If patient care is involved: In-service training: Clinical Research Billing and costs for patient care services Suzan Bruce, Coder, Clinical Trials Highly advised Highly advised Highly advised Activity #2. Licenses and Approvals Learning objective: Receive necessary approvals. Links NIH State Foundations Determine PI eligibility (exception to policy is required) If not eligible, submit Exception to Policy Form (105A) to SOM Sponsored Programs Radiation Use Authorization Contact Office of Sponsored Programs (703-9139) mandatory mandatory mandatory http://www.research.ucdavis .edu/f/f#contract-grantadministration mandatory Mandatory mandatory http://safetyservices.ucdavis .edu/ Mandatory (if applicable) Mandatory (if applicable) Mandatory (if applicable) Biosafety Use Authorization http://safetyservices.ucdavis .edu/ Mandatory (if applicable) Mandatory (if applicable) Mandatory (if applicable) Carcinogen Use Authorization http://safetyservices.ucdavis .edu/ Mandatory (if applicable) Mandatory (if applicable) Mandatory (if applicable) Human Anatomical Specimen Approval http://www.research.ucdavis .edu/u/a/rci Mandatory (if applicable) Mandatory (if applicable) Mandatory (if applicable) Human Stem Cell Approval http://www.research.ucdavis .edu/u/a/rci Mandatory (if applicable) Mandatory (if applicable) Mandatory (if applicable) Recombinant DNA Authorization http://safetyservices.ucdavis .edu/programs-andservices/biosafety/Forms%2 0and%20Plans/bua/biologic al-use-authorization-bua Mandatory (if applicable) Mandatory (if applicable) Mandatory (if applicable) Activity #3. Grant Development Learning objective: Links NIH State Foundations Download Grant program and guidelines (RFA) See agency website mandatory mandatory mandatory Notify Dept./ORU grants administrator of intent to submit Notify OVCR of intent to submit Department or ORU provides proposal support mandatory mandatory mandatory n/a mandatory n/a Download Application materials (forms) See agency website mandatory mandatory mandatory Request OVCR RFA review proposals@ucdavis.edu n/a mandatory n/a Understand guidelines for submission See agency website mandatory mandatory mandatory Contact CTSC for costs of patient care services, costs of monitoring/QA services (if human subjects involved) Contact CTSC for Protocol Analysis: data management, biostat, community involvement If Patient Care is involved: Learn about clinical research billing http://www.ucdmc.ucdavis.ed u/ctsc/ Highly advised Highly advised Highly advised http://www.ucdmc.ucdavis.ed u/ctsc/ Highly advised Highly advised Highly advised http://ucdmc.ucdavis.edu/clin icaltrials/BudgetingBilling/in dex.html Highly advised Highly advised Highly advised Activity #4. Prepare proposal Packet Learning objective: Common proposal component and guidances can be found at: http://www.research.ucdavis.edu/pgc/pp/cpc. The information and templates are intended to guide and assist you throughout the proposal preparation process. Please note that components vary between sponsors and sponsors periodically revise requirements. It is important to check the sponsor’s guidelines to ensure the most recent guidelines are followed. Consult OVCR Proposal Submission Checklist http://www.research.ucdavis.edu/pgc/d/spo/proposalsubmission-checklist Links NIH State Foundations OVCR Datasheet http://www.research.ucda vis.edu/f/f#contract-grantadministration mandatory mandatory mandatory Signature/Cover Page http://research.ucdavis.ed u/pgc/d/spo/samplecoverp age.pdf/view n/a n/a Draft proposal/Scope of Work See Agency Requirements Highly advised but not required at this point Highly advised but not required at this point Mandatory when sponsor does not provide a required cover page form Highly advised but not required at this point mandatory for final proposal mandatory for final proposal mandatory for final proposal See Agency Requirements mandatory mandatory mandatory See Agency Requirements mandatory mandatory mandatory Final Scope of Work Budget: • • • • • • • • • • • • % effort of all personnel Salary Benefits Equipment Consultants Travel Supplies Lease Graduate Student Fee remission Indirect Costs Subcontract Budget Indirect Costs on subcontract Budget Justification Conflict of Interest Disclosures for key personnel 800-U/ 700-U http://www.research.ucda vis.edu/f/f#contract-grantadministration Mandatory (800U) n/a Mandatory 700U Limited Submission selection documentation Notification letter from OVCR mandatory n/a mandatory SOM Transmittal Sheet http://www.ucdmc.ucdavi s.edu/medresearch/medsp/ forms.html mandatory mandatory mandatory Signed PI Eligibility Form /Exception to Policy, if required http://www.research.ucda vis.edu/pgc/pp/ps/pr/pr#pi -eligibility Mandatory Mandatory mandatory List of Key personnel See Agency requirements mandatory mandatory mandatory Biosketches of key personnel See Agency Requirements Will be required for final proposal Will be required for final proposal Will be required for final proposal Current and pending Support of key personnel See Agency Requirements Will be required for final proposal Will be required for final proposal Will be required for final proposal Facilities and Resources See Agency Requirements Will be required for final proposal Will be required for final proposal Will be required for final proposal Letter of Intent to Form Consortium (if subcontract) See OVCR Subcontract requirements http://www.research.ucda vis.edu/u/a/spo mandatory mandatory mandatory Subcontract Information • Face Page (398 form) • Scope of work • Budgets • Budget Just • List of Key Personnel • Biosketches • Facilities and Resources • Budgets See Agency Requirements mandatory mandatory mandatory Copy of Sponsor guidelines mandatory mandatory mandatory PI signature, Dept Chair Signature mandatory mandatory mandatory Primate Center Signature on OVCR Datasheet Mandatory, if applicable Mandatory, if applicable Mandatory, if applicable Activity #5. Submit to SOM Sponsored Programs Links NIH State Foundations Hard copy of the Proposal Packet from Step 4 submitted to SOM Sponsored Programs SOM Sponsored Programs reviews for PI eligibility and budgets: • Indirect cost rate • Cost sharing • Equipment • Gradual students fee remission • Consultants • Lease • Subcontracts • RFA mandatory mandatory mandatory mandatory mandatory mandatory SOM Sponsored Programs makes corrections as necessary mandatory mandatory mandatory Dean’s Signature mandatory mandatory mandatory Activity 6.Submit to OVCR Learning Objective: What OVCR will review and not review is specified on the OVCR website: http://www.research.ucdavis.edu/pgc/pp/ps/pr Links NIH State Foundations Department submits to OVCR by using either: • Hard Copy (original+1) • Edocs proposals@ucd avis.edu mandatory mandatory mandatory OVCR reviews: (for checklist list for specific review criteria see OVCR website) http://www.rese arch.ucdavis.ed u/pgc/d/spo/pro posalsubmissionchecklist mandatory mandatory mandatory Makes corrections if necessary mandatory mandatory mandatory OVCR Signature on behalf of the Regents on the OVCR Datasheet mandatory mandatory mandatory Study team gets the copy of approval to submit the application Finalize scientific component mandatory mandatory mandatory mandatory mandatory mandatory Finalize Biosketches, and Facilities and Resources mandatory mandatory mandatory Assemble Final proposal mandatory mandatory mandatory mandatory mandatory mandatory Submit to granting Agency: • Via OVCR electronically • Hard Copy via FedEx http://www.rese arch.ucdavis.ed u/edocs See Agency Guidelines for Submission Activity #7. Granting Agency Activities Receives the proposals and requests information (Justin-time). OVCR reviews proposal Granting Agency review of Just in time information (with restrictions, if applicable) PI completes IACUC/IRB approvals Links NIH State Foundations See granting agency requirements Mandatory Mandatory mandatory n/a Mandatory n/a Mandatory, if applicable Mandatory, if applicable Mandatory, if applicable Mandatory, if applicable Mandatory, if applicable Mandatory, if applicable Highly advised Highly advised Highly advised Mandatory, if applicable Mandatory, if applicable Mandatory, if applicable recommended recommended recommended Mandatory Mandatory mandatory http://research.ucdavis .edu/c/cs/hrp http://safetyservices.uc davis.edu/IACUC If human subjects involved: contact CTSC for IRB submission services, clinical research services, clinical research billing and monitoring/QA services Granting Agency issues notice of award http://www.ucdmc.ucd avis.edu/clinicaltrials/ Forinvestigators/forinv estigators.html Request to expend funds prior to award http://www.research.u cdavis.edu/pgc/d/spo/ AdvanceAcctRequest. pdf OVCR issues approval to expend funds through Extramural Acct Activity 8. Set up Accounts Links NIH State Foundations Extramural Accounting opens OP Fund http://accounting.ucda vis.edu/EX/newaccoun ts.cfm mandatory mandatory mandatory Financial Analyst establishes DaFIS account http://accounting.ucda vis.edu/EX/newaccoun ts.cfm mandatory mandatory mandatory Activity #9. Grant Maintenance Learning Objective: To understand the steps after the approval for a study is granted and the Investigator is ready to start enrolling subjects. Financial Compliance / monthly financial review Initiate Effort and Cost Recovery Generate and Submit yearly Progress Reports Keep Current: authorizations and approvals If human subjects are involved: Follow Clinical Research Checklist and Process Maps Links http://accounting.ucdavi s.edu/EX/fincompliance .cfm http://intranet.ucdmc.uc davis.edu/ctsc/area/clini caltrials/processmaps.sh tml NIH mandatory State mandatory Foundations mandatory mandatory mandatory mandatory mandatory mandatory mandatory mandatory mandatory mandatory mandatory mandatory mandatory Activity #10. Grant Close out Learning objective: Award Closeout Checklist Links http://accounting.ucdavis.edu /EX/AwardCloseoutChecklis t.pdf NIH mandatory State mandatory Foundations mandatory Submit final report to the agency See granting agency requirements mandatory mandatory mandatory Financial Closeout http://accounting.ucdavis.edu /EX/fincompliance.cmf mandatory Mandatory mandatory If human subjects are involved: Follow Clinical Research Checklist and Process Maps http://intranet.ucdmc.ucdavis .edu/ctsc/area/clinicaltrials/pr ocessmaps.shtml mandatory mandatory mandatory