BioBrick Assembly Methods Standard assembly In-Fusion PCR assembly
advertisement

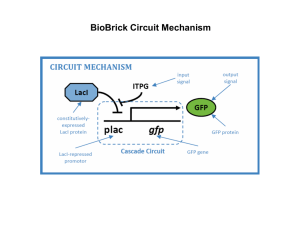
BioBrick Assembly Methods Standard assembly In-Fusion PCR assembly Using standard assembly to construct a simple GFP circuit BioBrick Circuit Mechanism BioBrick Circuit Plasmid Standard Assembly Overview 1. Amplify R and E BioBrick plasmids by transformation into a cloning strain 2. Pick a single colony for overnight growth 3. Isolate plasmids using a miniprep kit 4. Run plasmids on a gel 5. Restriction digest of plasmids 6. Run digests on a gel and isolate the fragments of interest 7. Ligate fragments together to form the engineered plasmid 8. Transform plasmid into an expression strain expressing lacI 9. Induce GFP expression with IPTG 10. Measure GFP fluorescence with plate reader or fluorescence microscope Transformation via electroporation Successful Transformants (plated on LB + Amp plates) http://www.umext.maine.edu/onlinepubs/htmpubs/images/growcurve.gif Overnight cultures (grown in LB + Amp media) First step of a miniprep: pellet cells with centrifugation Rules of Gel Electrophoresis • DNA runs towards the positive pole • Smaller DNA fragments travel faster than larger ones • Electrophoretic mobility from slowest to fastest: nicked open-circular, relaxed circular, linear, supercoiled (covalently closed-circular), supercoiled denatured E-gel Purified BioBrick plasmids 1 = Purified R plasmid (Sean) 2 = Purified E plasmid (Sean) 3 = Purified R plasmid (Alec) 4 = Purified E plasmid (Alec) M = E-gel ladder 5 = 1 kb ladder BioBrick Circuit Assembly BioBricks Plasmid Digest 1 = 1 kb ladder 2 = Digested R plasmid (Sean) 3 = Undigested R plasmid 4 = Digested R plasmid (Alec) M = E-gel ladder (10 uL) 5 = E-gel ladder (25 uL) 6 = Digested E plasmid (Sean) 7 = Undigested E plasmid 8 = Digested E plasmid (Alec) Next we need to ligate the R-vector with the E-insert with DNA Ligase Ligation of R and E BioBricks ACTAGT TGATCA XbaI Mixed SpeI A TGATC ACTAGA TGATCT CTAGA T PstI PstI CTGCAG GACGTC TCTAGA AGATCT TGCAG C CTGCAG GACGTC C GACGT CTGCAG GACGTC Measuring DNA concentration for ligation reaction We want 10 ng of vector mass. How much insert mass do we need? Calculation of R BioBrick Restriction R BioBrick: Cut with S & P = 2079 – 14 + 55 = 2120 bp SpeI PstI EcoRI XbaI Calculation of E BioBrick Restriction E BioBrick: Cut with X & P = 6 + 876 + 16 = 898 bp SpeI PstI EcoRI XbaI Measuring DNA concentration for ligation reaction We want 10 ng of vector mass. How much insert mass do we need? R-vector = 7.5 ng/ul E-insert = 18.4 ng/ul 25.4 ng E-insert = 6 X (898 / 2120) X 10 ng R-vector 10 ng R-vector / 7.5 ng/ul = 1.33 ul 25.4 ng E-insert / 18.4 ng/ul = 1.38 ul Expression strain That expresses lacI Successful Transformants Also, it is essential to miniprep the plasmid and sequence it. GFP-expressing colonies Fluorescence microscopy photo of R0011+E0240 circuit in E. coli induced with IPTG