Bio1A Quiz 1 Study Guide
advertisement

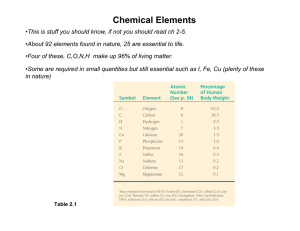
Bio1A Quiz 1 Study Guide Lecture 1 – Introduction to Molecular Biology A. Module 1: Evolutionary Principles a. Evolution of Cells i. All organisms made from cells (Fig. 2) 1. Prokaryotic – simple, no membrane-bound organelles 2. Eukaryotic – complex, has membrane-bound organelles ii. DNA exists in all cells. They may be packaged differently. iii. Membranes are all the same (Fig. 11) b. Unity and Diversity i. Homologous structures – different function/structure but common ancestor (Fig. 9) ii. Changes in DNA explain diversity. Genetic code of DNA is the same in all organisms (Tab. 1) iii. Energy usage is virtually the same in all organisms. Breaking ATP releases energy (Fig. 12) iv. Reproduction c. Evolutionary relationships can be described by “trees”. (Fig. 14) B. Module 1: Biological Information a. Genetic information is stored in nucleic acids i. DNA bases are A, T, G, C. Their sequence provides information (Fig. 10a) b. Information flow in a cell i. The “Central Dogma” states information is expressed by being passed from DNA RNA Protein. Protein is the functional part of this information (Fig. 10b) c. Heredity i. Genetic information is passed down to the next generation of cells. DNA replication allows this. DNA has evolved to be a great molecule for this. C. Module 3: Practicing Science a. Scientific Method: Question (Observation) Hypothesis Experiment Conclusion (Fig. 3) i. Hypothesis is an answer or prediction to the question. It must be falsifiable. ii. Experiment includes: design, data collection, and evaluation. 1. Controls (control variables) – factors held constant for verification of results. 2. Independent variable – factor manipulated by researcher. 3. Dependent variable – what is measured by researcher. iii. Conclusion accepts or rejects hypothesis. b. Reductionism vs. systems approach. i. Reductionism – break down mechanism to simplest components ii. Systems – looks at relationship of components with the whole organism. Bioinformatics with computers allows this approach today. c. Social Context: the use of powerful technologies, ethics, social responsibility Lecture 2 – Chemistry of Water and Acids/Bases A. Module 6: Properties of Water a. Water is a polar molecule with a slight positive charge on the hydrogen atoms and slight negative charge on the oxygen atom. This allows hydrogen bonding between water molecules and other molecules. (Fig. 1, 2) i. Hydrophilic – water loving molecules ii. Hydrophobic – water fearing molecules (non-polar) b. Heat sink. i. High specific heat. Water requires much heat energy to raise its temperature, and releases heat slowly; this helps maintain a constant body temperature. ii. Evaporation. Water requires heat when changed to vapor; sweating therefore cools animals rapidly. c. Cohesion/Adhesion. Water columns allow transport in organisms (Fig. 4) d. Solvent. Dissolver is solvent, dissolved is solute. (Fig. 5) e. Liquid. Hydrogen bonds form and break at equilibrium. Lower temperature lower kinetic energy so water freezes. Structure of ice and water (Fig 8) B. Module 7: Acids and Bases a. Water can dissociate into equal numbers of hydrogen ions and hydroxide ions. i. H2O H+ + OHii. In reality: 2 H2O H3O+ + OH- (Fig. 1) b. pH Scale. Defined as negative logarithm of the hydrogen ion concentration; neutral water dissociates into 10-7 moles/liter of hydrogen ions. pH = - log [H+]. Log is a ten-fold scale. (Fig. 2) i. Acids contribute to hydrogen ion when dissolved (Fig. 3, 4) ii. Bases contribute hydroxide ion, or remove hydrogen ion when dissolved (Fig. 5) c. Buffers resist pH change. (bicarbonate) HCO3- + H+ H2CO3 (carbonic acid) in blood. (Fig. 6) d. Ocean acidification is detrimental to coral reefs. (Fig. 7) Lecture 3 – Organic Chemistry A. Module 5: Basic Principles of Organic Chemistry a. Carbon i. Organic molecules contain carbon. ii. Carbon is tetravalent b. Hydrocarbons are backbones for many organic compounds: double/triple bonds, rings, branching, etc. (Fig. 5) c. Isomers (Fig. 6) i. Structural – different covalent partners ii. Geometric – different arrangement across double bond iii. Enantiomer – handedness. e.g. amino acid and ibuprofen d. Important functional groups (Fig. 8) i. Hydroxyl: -OH polar, reactive ii. Carbonyl: C=O polar iii. Carboxyl: COOH polar, reactive, ionizing (acid) iv. Amino: NH2 polar, reactive, ionizing (base) v. Sulfhydryl: SH polar, reactive vi. Phosphate: PO42- polar, reactive, ionizing (acid) vii. Methyl: CH3 nonpolar e. Macromolecules i. Monomer polymer ii. Most formed by dehydration synthesis iii. Broken by hydrolysis iv. Bonds 1. Ether: bond formed from two alcohols 2. Ester: bond between alcohol and carboxylic acid f. Stanley Miller Experiment shows possible synthesis in prebiotic conditions (Fig. 10) B. Module 8: Carbohydrates a. Functions: energy, structure b. Structure i. Monosaccharides have general structure of (CH2O)n ii. Linear forms have hydroxyl and carbonyl groups (Fig. 1) iii. Ring form is used to create polymers (Fig. 6) c. Examples of polysaccharides (Fig. 10) i. Cellulose has beta 1-4 linkages that are difficult to break. ii. Starch/glycogen have alpha 1-4 linkages that are breakable. iii. Chitin has decoration to make it hard. C. Module 9: Lipids a. Functions: energy, insulation, membranes, signaling b. Structure i. Largely nonpolar c. Examples i. Fatty acids – hydrocarbon chains usually containing 16 - 18 carbons and ending with an acid group. (Fig. 6) 1. Saturated have no double bonds (Fig. 8) 2. Unsaturated have double bonds (Fig. 9) ii. Triacylglycerols: formed by one glycerol reacting with three fatty acid molecules. (Fig. 7) iii. Phospholipids. 1. Two fatty acids linked to glycerol. One phosphocholine on last –OH of glycerol. Has hydrophilic and hydrophobic regions = amphipathic. (Fig. 1) 2. Forms various structures in water (Fig. 3) 3. Membranes are lipid bilayers. (Fig. 4) iv. Steroids – e.g. cholesterol, stiffens membranes and precursor to hormones (Fig. 5) D. Module 10: Proteins a. Diverse and numerous functions (Tab. 1) b. Structure i. Amino acid peptide 1. Amino acid (Fig. 1) 2. R groups – 3 types (polar, non-polar, charged), 20 amino acids (Fig. 2). 3. Peptide bond (Fig. 3) 4. N-C termini ii. Levels of structure (Fig. 6) 1. Primary – linear amino acid sequence 2. Secondary – internal folding. Examples: beta-sheets, alpha-helices. Uses almost exclusively hydrogen bonds (Fig. 4) 3. Tertiary – final structure. Uses more bonds (e.g. hydrogen, ionic, covalent, hydrophobic) (Fig. 5) 4. Quaternary – if there is more than 1 peptide 5. Sickle-Cell Anemia is an extreme example of what can go wrong with structure. iii. Conformation 1. Naturation and denaturation 2. Factors: temp, pH, salt, other proteins E. Module 12 – Nucleic Acids a. Functions: hereditary information, energy molecule, coenzymes, signaling. b. Structure i. Nucleotides are made of a phosphate, a pentose sugar, and a nitrogenous base. PSugar-Base. Nucleoside is without phosphate. (Fig. 2, 9) ii. Bases are purines (double ring) and pyrimidines (Fig. 1) iii. Linkage is phosphodiester bond: P-sugar-P-sugar etc. iv. 4 bases for DNA, 4 (and more) for RNA 1. Base pairing rules A=T, G=C (Fig. 4) 2. For RNA A=U, G=C v. DNA and RNA differences 1. 2’OH on RNA 2. DNA exists as double helix (Fig. 5) while RNA is single-stranded but binds to itself (Fig. 7) Lecture 4 – Energy and Enzymes A. Module 2: Energy - ability to do work a. Types of Energy i. Kinetic (motion) vs. potential (stored) ii. Chemical is potential energy iii. Free energy – available energy in a system to do work b. Types of chemical reactions (Fig. 2) i. Endergonic – requires energy ii. Exergonic – releases energy iii. Coupled – use energy from an exergonic to drive an endergonic (e.g. ATP) c. Metabolism – the sum of all reactions in a cell i. Catabolism – breakdown molecules ii. Anabolism – build iii. Reactions tend to be organized in pathways B. Module 11: Enzymes a. Enzymes are catalysts that lower energy of activation (Fig. 1, 2) b. Enzyme i. Specific for certain substrates ii. Reaction must already be exergonic iii. Reusable c. Substrate Product d. Catalytic cycle: E + S ES EP E + P (Fig. 3) i. Substrate binding: binds to active site of enzyme. Uses “induced fit” model instead of “lock-and-key”. ii. Reaction: active site provides correct environment to create product. iii. Release: enzyme releases product and can be reused. e. Regulation i. Environmental factors 1. Optimal pH, temperature, salt produce bell curves (Fig. 4) 2. Substrate concentration (Fig. 5) ii. Activators 1. Cofactors (non-organic) 2. Coenzymes (organic) iii. Inhibitors (Fig. 6) 1. Competitive – binds active site 2. Noncompetitive – binds allosteric site iv. Complex Regulation 1. Feedback inhibition 2. Enzyme complexes a. Allosteric activation and inhibition as a complex b. Cooperativity – when substrate binding assists further substrate binding of other enzymes in complex 3. Localization – enzymes are held in separate compartments