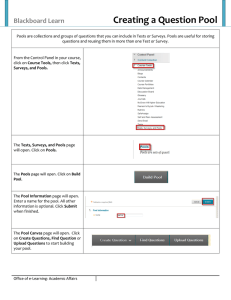
Claudia Rafael Mariano Baigun for the degree of Master of...
advertisement

AN ABSTRACT OF THE THESIS OF Claudia Rafael Mariano Baigun for the degree of Master of Science in Fisheries Science presented on November 14. 1994. Title: Characteristics of Pools Used by Adult Summer Steelhead (Oncorhynchus mykiss) in the Steamboat Creek Basin. North Umpgua River, Oregon. Redacted for privacy Abstract approved: James R. Sedeil This study examined features of deep pool (>0.8 m mean depth) used by adult summer steelhead in Steamboat Creek (1991-1992). Steamboat Creek had a heterogenous thermal profile, with some segments exceeding preferred temperature of steelhead. Deep pools were scarce (4 % of the total habitat units) and 39 % of them were identified as cool pools (mean bottom water temperature 19°C). Adult summer steelhead were found primarily in deep pools, avoiding other habitats (glides,riffles) and even cold but shallow tributary junctions. Use of odds ratio showed that use of cool pools use was estimated to be 11 times greater than the odds of the use of warm pools (P <0.001). Discriminant analysis identified mean bottom pool water temperature, riparian forest at the pool bank, proportion of large boulders, maximum length and mean depth as the best subset of variables that accounted for differences between pools occupied and not occupied by adult steelhead. A total of 69 % of the variation was explained by differences in used and not used groups. Classification accuracy was 89 %. Canton Creek, a tributary of Steamboat Creek, were tested as validation site for the derived model, observing that the classification function performed moderately, achieving a hit-ratio of 0.7. Results of the study showed that, since bottom pool temperature was a major factor but other ecological factors were also relevant, an integrated framework would be required in determining pool used by this species. Moderate success of the predictive model suggests that managers will want to check it before applying in other basins. @copyright by Claudio Rafael Mariano Baigün November 14, 1994 All Rights Reserved Characteristics of Pools Used by Adult Summer Steelhead (Oncorhynchus mykiss) in the Steamboat Creek Basin, North Umpqua River, Oregon by Claudio Rafael Mariano BaigCin A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Completed November 14, 1994 Commencement June 1995 Master of Science thesis of Claudio R. M. BaigUn presented on November 14. 1994 APPROVED: Redacted for privacy Professor, representing Fisheries Science Redacted for privacy , Head of Deiartment of Fish and Wildlife Redacted for privacy Dean of GradIath School I understand that my thesis will become part of the permanent collection of Oregon State University libraries. My signature below authorizes release of my thesis to any reader upon request. Redacted for privacy Claudlo Rafael Mariano Baigün ACKNOWLEDGMENT This section is not necessarily extensive but pleasant. Several people and institutions have been involved in this research. Without their support this dissertation would not have been completed. I want to thank the personnel of the Department of Fish and Wildlife (OSU), in particular Chris Sennet, Jean Smith, Charlotte Vickers and the past Department Chairman, Dr. Richard Tubb, for providing me with a comfortable and friendly work atmosphere. I am deeply indebted to the U.S. Forest Service, Pacific Northwest Research Station (PNW), CorvalUs, for assistance with computer and other facilities. Bruce Hansen provided me with all the necessary support and helped to improve my field work. Cathie Quinn, Joe Lumianski, Mark Chambers and, particularly, Mark Garner assisted me in the field and I want to thank them for their help. Barbara Fontaine and Tim La Marr from the Umpqua National Forest, were friendly people who became interested in my research, providing facilities and the necessary support in the Steamboat Creek area. Jeffry Dambacher, Oregon Department of Fish and Wildlife, provided data of fish number at Winchester Dam. Dr. Terry Roelofs allowed me to analyzed his unpublished data from Steamboat Creek basin and also provided me with valuable bibliographic material. Kevin McGarigal provided statistical assistance. Other people, not directly related with the project also provided valuable aid: Paula Carter from the International Institute of Education (lIE, San Francisco) and Marybeth Rowen from the Office of International Education (OSU). Partial financial support were provided by the Fulbright Commission (Argentina) and Encyclopedia Britannica Fellowship. Special thanks to Dr. Jim Sedell, my advisor professor for many reasons. First, he provided funds for my research and training program. Second, Jim provided vital guidance for this study and contributed greatly to the development of my research ideas. Third, and perhaps what is most relevant to me, he took the risk of accepting me as part of his research team, without any prior knowledge of my person, and deposited his confidence in my work. I hope I did not disappoint him. I wish also to thank Dr. Carl Schreck, Dr. J. Lyford and Dr. Gordon (Gordie) Reeves who served on my graduate committee, and Dr. Myron Schenk who served as graduate council representative. Gordie spent much time discussing this study with me this study and bravely answered the challenge to correct earlier drafts of this thesis. Dr. Stan Gregory also made valuable suggestions. Special thanks to our big family in Argentina (the Leff-Baigün-Minotti team), who supported us in different ways and have patiently awaited our return, and of course, to my family here in Corvallis. I dedicate this thesis to them. To Luci and Ale, for accepting that I used much of their time for my work. To my lovely wife Priscilla, who deserves enormous and invaluable credit for the completion of this thesis. Her natural talent for ecology, that surpasses mine, was a precious source of rich ideas and original suggestions. Thanks for all 'Patil!. TABLE OF CONTENTS I.INTRODUCTION 1.1. Objectives 3 II. MATERIAL AND METHODS 2.1. Description of the study area 2.2. Data collection 4 4 6 2.2.1. General sampling framework 2.2.2. Environmental sampling 2.2.2.1. Definition of deep pools 2.2.2.2. Deep pooi sampling 2.3. Basis for selected variables 2.4. Definition of cool and warm pools 2.5. Fish sampling 2.6. Statistical analysis Ill. RESULTS 3.1. Steamboat Creek thermal regime 3.2. Distribution of deep pools 3.3. Pool characteristics 3.3.1. Morphometry 3.3.2. Water temperature 3.4. Fish abundance and distribution 3.4.1. Systematic sampling 3.4.2. Pool sampling 1 7 10 10 11 15 17 18 20 25 25 28 28 28 30 32 32 32 3.5. Temperature-abundance relationship 3.6. Relationship between poo1 environmental and pool use 36 3.6.1. Selection of predictive variables 39 3.7. Pool use prediction 3.7.1. Square generalized distance 3.7.2. Development of a field key 39 44 44 45 TABLE OF CONTENTS (Continued) IV. DISCUSSION 48 4.1. Thermal characteristics of the system 4.2. Fish distribution patterns 48 49 4.2.1. Use of cool and warm pools 50 4.3. Discriminant analysis 4.4. Validation of the discriminant model 4.5. Effects of population size 51 55 56 V. MANAGEMENT CONSIDERATIONS AND FUTURE DIRECTIONS 5.1. Deep pools protection 5.2. Considering water temperature as a source of pool complexity 5.3. Model testing 5.4. Conduct studies on deep pool thermal dynamic 5.5. Evaluate the stream thermoregulatory capacity 5.6. Study of fish movements 5.7. Considering the riverscape perspective in deep pool use evaluation 58 59 BIBLIOGRAPHY 69 APPENDICES Appendix A Appendix B 60 61 62 63 64 65 87 88 92 LIST OF FIGURES Figure 1: Steamboat Creek basin location in Oregon and expanded view. 5 Figure 2: Steamboat Creek and main tributaries. 8 Figure 3: Canton Creek and main tributaries. Figure 4: Distribution of deep pools (mean depth > 0.8 m) in Streamboat Creek, OR, sampled in this study. 12 Figure 5: Distribution of deep pools (mean depth > 0.8 m) in Canton Creek, OR, samp(ed in this study. 13 Figure 6: Thermal profile of Steamboat Creek. Zero point corresponds to the junction with the North Urnpqua River. Arrows indicate main tributary junctions. CA: Canton Creek; S: Steethead Creek; R: Reynolds Creek; BB: Big Bend Creek; C: City Creek. 26 Figure 7: Distribution of cool pools ( 19°C mean bottom water temperature) and warm pools (> 19°C mean bottom water temperature) in Steamboat Creek. Pools of similar category that were located close together are shown as only one point. Zero point corresponds to the junction with the North Umpqua River. 32 Figure 8: Mean number of adult summer steelhead in deep pools in Steamboat Creek during August-September 1991 and August-September 1992 (see figures 4 an 5 for pool locations). Note: pools 1-10 are located in Canton Creek (see Fig. 3 for pool locations). 35 Figure 9: Relationship between mean water temperature at the bottom of the pool and number of adult summer steelhead in study pools in Steamboat Creek in A: August-September 1991; B: August -September 1992. 37 Figure 10: Plot of discriminant scores based on analysis in Steamboat Creek. Vertical lines on the block and the circle respectively indicate the centroid position. Horizontal line at the extremes corresponds to the ranges. 43 LIST OF FIGURES (CONTINUED) Figure 11: Number of adult summer steelhead at Winchester Dam (North Umpqua River), counted between May and November (1969-1992)(Oregon Fish and Wildlife Department, unpublished data). 58 LIST OF TABLES Table 1: Preferred water temperature of rainbow trout reported in the literature. L.N.A: location not available. 19 Table 2: Water temperature (°C) of Steamboat Creek tributaries. Numbers in parenthesis represent the water temperature in Steamboat Creek above the junction with the respective tributary. Temperatures were measured between 1 pm and 4 pm. Tributaries are ordered in an upstream-downstream position. 27 Table 3: Statistical parameters of measured variables in the study pools in Steamboat Creek, OR, in 1991 and 1992. 29 Table 4: Correlation matrix among physical features of deep pools in Steamboat Creek, OR, summer 1991-1992. LED: ledge cover proportion; S:sand proportion; BR: bedrock proportion; LB: large boulder proportion; C: cobble proportion; SB: small boulder proportion; SO: small gravel proportion; LG: large gravel proportion; A: Area; VOL: volume; MD: mean depth; MAD: maximum depth; OR: depth ratio; MW mean width; LIT: littoral area proportion; ML: maximum length; SSI: shore sinuosity index; BO: bottom oxygen; TVC: temperature variation coefficient; BT: mean bottom pool temperature (see text for further explanation). 31 Table 5: Total number of adult summer steelhead in cool and warm pools in Steamboat Creek during August-early September 1991 and 1992. (S.D: standard deviation). 33 Table 6: Percent of cool pools occupied by adult summer steelhead adults in Steamboat Creek, percent of cool pools having and mean density (fish/rn3) in cool pools (6) warm pools (6w) and all deep pools (6) during 1991 and 1992. Table 7: Results of odds ratio test of pools with different mean bottom water temperatures that were use and not used by adult summer steelhead in Steamboat Creek, OR, August-September 1991 (A) and August-September 1992 (B). See text for further detail. 34 38 LIST OF TABLES (CONTINUED) Table 8: Mean. standard deviation (S.D.) and range of variables between USED and NOT USED pools by adult summer steelhead in Steamboat Creek, OR, August-September 1991 and 1992. See text for further detail. RF: proportion of bank riparian forest; LB: large boulder proportion; MD: mean depth (m); ML: maximum length (m); BT: mean pool bottom temperature (°C). 40 Table 9: Standarized canonical coefficients and structure coefficients derived from discriminant analysis. 41 Table 10: Standarized canonical coefficients and structure coefficients derived from discriminant analysis. 41 Table 11: Number of pools in Steamboat Creek correctly classified by discriminant analysis after jacknife resampling procedure. 44 Table 12: Number of pools in Canton Creek correctly classified by discriminant analysis. 45 LIST OF APPENDIX FIGURES Figure A.1: Number of fish observed in pools during the systematic survey in Steamboat Creek (August 1991). 89 Figure A.2: Conceptual example of the dichotomous key development according to Kendall (1975). See text for further explanations. 90 Figure A.3: Proportion of habitat types in Steamboat Creek observed in the systematic survey during 1991. 91 LIST OF APPENDIX TABLES Table B.1: Percentages of bank riparian forest in pools USED and NOT USED by adult summer steelhead in Steamboat Creek, as utilized in the development of the dichotomous key. Dashed lines defines cut-off values. 93 Table B.2: Maximum length in pools USED and NOT USED by adult summer steethead in Steamboat Creek, as utilized in the development of the dichotomous key. Dashed lines defines cut-off values. 94 Table B.3: Mean bottom temperature in pools USED and NOT USED by adult summer steelhead in Steamboat Creek, as utilized in the development of the dichotomous key. Dashed lines defines cut-off values. 95 Table B.4: Maximum depth in pools USED and NOT USED by adult summer steelhead in Steamboat Creek, as utilized in the development of the dichotomous key. Dashed lines defines cut-off values. 96 Table B.5: Comparative analysis of physical variables in pools in Canton Creek and Steamboat Creek. X= mean; SD= standard deviation; n.s: not significative at 0.05 level; s: significative at 0.05 level; power= 1- Type II error. 97 Table B.6: Values measured in Steamboat Creek basin pools. AREA: area (m2); VOL: volume (m3) ZD: mean depth (m); ZX: maximum depth (m); DR: depth ratio; MW mean width (m); LIT: littoral area proportion; PER: perimeter (m); ML; maximum length (m); SSI: shore sinuosity index; LED: ledge cover proportion; RF: proportion of bank riparian forest; S:sand proportion; BR: bedrock proportion; LB: large boulder proportion; SB: small boulder proportion; C:cobble proportion; SG: small gravel proportion; LG: large gravel proportion; BO: bottom oxygen (mg/L); TCV: temperature coefficient of variation; BT: mean bottom temperature (°C) (see text for further explanation). 99 Characteristics of Pools Used by Adult Summer Steelhead (Oncorhynchus mykis.$) in the Steamboat Creek Basin, North Umpqua River, Oregon I. INTRODUCTION Pools are important habitat for juvenile and adult salmonids. They offer cover, protection from predators and rearing and holding opportunities (Binns 1982; Oswood and Barber 1982; Campbell and Neuner 1985; Platts et al. 1983, 1987; Beschta and Platts 1986; Sedell et al. 1986; Modde et at. 1991; Reeves et aL 1991; Faush and Northcote 1992; Northcote 1992). Factors such as pool area, volume, cover and depth influence the density and biomass of salmonids in pools (Lewis 1969; Rinne 1982; Plalls et at. 1983; 1987; Raleigh et aL 1984; Bowlby and Roff 1986; Wesche et at. 1987). Water temperature can influence pooi use by salmonids (Bowiby and Roff 1986; Berman and Quinn 1991; Nielsen 1992: Nielsen et at. 1994). Temperature is the dominant factor that controls the behavior and activities of aquatic poikiloterms (Stauffer 1980). In general, temperature is a pervasive factor that affects several physiological processes, such as metabolism, diseases susceptibility, growth and feeding (Lantz 1970; Adams et at. 1990; see also Beschta et al. 1987 for a review). Salmonids may seek cooler pools as a mechanism for thermoregulation (Kaya et al. 1977; Clapp and Clark 1990; Meisner 1990; Berman and Quinn 1991), and residing in cool thermal refuges may allow 2 reported that steelhead inhabiting streams above 21 °C may have difficulties extracting oxygen from the water. These temperature constraints have been noted in different Pacific Northwest streams and rivers (Everest 1973; Dunn 1981; Nielsen 1992; Nielsen et al. 1994). Summer steethead (Oncorhynchus mykiss) ascend rivers from May to October and hold in pools in small to intermediate size streams until spawning between January and March (Leider 1985; Barnhart 1986). Since these fish spend all summer in freshwater, they may confront harsh ecological conditions particularly in some California and Oregon streams. Water temperature has been identified as a key ecological factor that influences adult summer steelhead in many streams in California and western Oregon (Anderson and Miyajima 1975), but this tenet has been supported by only a few quantitative studies (e.g. Adams et al. 1990; Nielsen 1992; Nielsen et at. 1994). On the other hand, ecological factors as depth and cover have been identified in the literature as affecting salmonid abundance or density in pools, but little effort has been made to determine the influence of these and other factors in pool use by adult steelhead (e.g. Dunn 1981; Freese 1982). An integrated framework is needed to describe the suite of factors and their relative importance in governing pool use by adult summer steethead. In the present study, I examined features of pools used by adult summer steelhead in a mid-size Oregon stream. My general hypothesis was that pools occupied by summer steethead are selected primarily on the basis of thermal characteristics. I also examined the relevancy of geomorphic variables in defining a second hierarchical level that accounts for the distribution of adult summer steelhead in pools. 1.1. Objectives The specific objectives were to: a) Determine the relationship between pools used by adult summer steelhead and the position of the pools in the thermal gradient of the stream. b) Determine if adult summer steelhead use pools with cooler water temperature more than pools with warmer water. C) Determine the relative importance of other ecological factors in pool use. ri II. MATERIAL AND METHODS 2.1. Description of the study area The Steamboat Creek basin is located in the west Cascades, Douglas County, Oregon (Fig. 1). The basin covers approximately 600 km2. Historical mean annual flow for Steamboat Creek near its confluence with the North Umpqua River has been reported as 20.48 m3/s, with a historical maximum of 1444 m3/s and a minimum of 0.85 m3/s (Hubbard et al. 1990). Annual precipitation in the basin averages 140 cm and 70 % of the precipitation occurs from November through March (Dambacher 1991). Steamboat Creek, a fifth order stream at its mouth, is the major stream in the basin. Canton and Big Bend Creeks are the most important tributaries (Brown et al. 1971). The basin has supported intensive timber harvest during the past 35 years, with 34% of the basin harvested between 1955 and 1990 (Holaday 1992). Timber harvest has modified stream temperatures, resulting in higher mean and daily high temperatures (Hostetler 1991). The Steamboat Creek basin supports a diverse fish community. Salmonids include summer and winter steelhead, resident rainbow trout (0. mykiss), sea-run and resident cutthroat trout (0. clarki), coho salmon (0. kisutch), chinook salmon (0. tshawytscha), brown trout (Sa/mo trutta) and brook trout (Salvelinus fontinalis). Other species include redside shinner (Richardsonius baleatus), suckers (Catostomus spp.), Umpqua long-nose dace (Rhinichthys evermanni), Umpqua Figure 1: Steamboat Creek basin location in Oregon and expanded view. Ii OHEGON I Roseburg North Umpqua Fliver I South Umpqua River I cr1 squawfish (Ptychochei/us umpquae), sculpins (Cottus spp.), speckled dace (Rhinichthys osculus) and juvenile Pacific lamprey (Lampetra tridentata) (Dambacher 1991). The basin has been recognized as the main spawning area for summer steelhead in the North Umpqua River basin (Collins 1971), and fishing has been closed since 1932 (Marlega 1971). Adult steelhead enter the Umpqua River from May until December (J. Dambacher pers. comm.). Only steelhead migrating from June to September are considered to be summer steelhead. Some of these fish hold in the Steamboat Creek basin until spawning, which occurs between December and March. Summer water temperatures in areas of Steamboat Creek basin where adult summer steelhead hold, may surpass the optimum physiological tolerance levels of steelhead (Brown et al. 1971; Holaday 1992). Low flows may reduce suitable resting and holding habitats. Previous studies on steelhead ecology in the area have been focused mostly on juvenile stages: density and distribution were studied by Roelof et al. (1983) and Dambacher (1991), species interactions by Reeves et al. (1987), and influence of instream structures by Fontaine (1987). Adult studies examined movements of adult summer ste&head (Wroble and Roelof 1985; Wroble 1990). 2.2. Data collection Steamboat Creek was selected as the primary study area. The study area extended from the junction of the North Umpqua River 32 km upstream to the 7 junction of the East Fork (Fig. 2). Canton Creek was also surveyed and habitat characteristics and fish presence in deep pools (see below) were used to validate results obtained in Steamboat Creek. For Canton Creek, the study area was from the junction with Steamboat Creek to the junction with Pass Creek, 15 km upstream (Fig. 3). In both streams, upper boundaries were considered the limits of anadromous salmonid distribution during the summer. 2.2.1. General sampling framework The sampling scheme was based on three stages: an initial systematic survey in Steamboat Creek during 1991 was made to determine habitat types and frequency and to identify characteristics of pool used by adult summer steelhead. Twenty four sites were established every 800 m, located between the junction of Steelhead Creek and the East Fork. Each site extended 200 m upstream from the sampling site boundary. Glides, riffles, cascades and pools were identified according to Helm (1985) and counted in the 200 m reach. The lower segment of Steamboat Creek was not surveyed because of field limitations. All units were sampled to determine the presence and abundance of adult summer steelhead. All pools with the features identified in the systematic survey were sampled for fish and selected physical features. In every case fish were sampled prior to measuring physical variables. In an attempt to frame a general picture of ecological factors that may govern the occupancy of the pools, I sampled the entire population of deep pools. Thus the sampling scheme emphasized sampling N I N tause 0 ___! n CJ -' Figure 3: Canton Creek and main tributaries c. 10 sites over observations per site (Matthews 1990). Such an approach was considered appropriate because past observations performed in the basin were focused only on a few pools (e.g. Roelof et al. 1983). 2.2.2. Environmental sampling 2.2.2.1. Definition of deep pools A frequent problem in the analysis of environmental characteristics and species abundance or presence is that some habitats are never used by species. In single species studies is important to maximize those areas where the species can be found (Bibby et al. 1992). In streams, physical limitations such as shallowness, lack of accessibility or low flow are factors that may constrain habitat availability and therefore the universe of potential sampling sites (see Gorman and Karr 1978; Bartholow and Slauson 1990). Thus, a preliminary analysis was conducted to identify those pools that did not hold adult fish. Using the systematic sampling as reference, pool length, mean width, surface, and mean depth were calculated and related to adult summer steelhead presence. A consistent pattern was evident with mean depth. Pools having a mean depth <0.8 m. never held adult fish and therefore these pools were defined as shallow pools. Pools m mean depth were defined as deep pools. 0.8 11 2.2.2.2. Deep pool sampling All deep pools were identified in Steamboat Creek and Canton Creek in August and September 1991. Thirty eight pools were located in Steamboat Creek (Fig. 4) and 10 deep pools were identified in Canton Creek (Fig. 5). Four pools in Steamboat Creek were not sampled because of poor visibility or human disturbances, such as swimming. Transects were established every 3 m along the length of the pool in those pools <20 m in length. Selected variables were measured every 2 m across a transect, beginning at the bank in these pools. Transects were established every 5 and 7 m in pools 20-40 m and in pools >40 m, respectively. Variables were measured every 3 m across a transect beginning at the bank in these pools also. The following characteristics were measured in each pool: a) Bank riparian forest, defined as the proportion of the pool bank occupied by trees and high shrubs (>3 m) that were twice as high as the distance to the water edge (Duff and Cooper 1976). b) Substrate composition, defined as the substrate type located below a sampling point. Categories were: sand (0.25-0.5 mm), small gravel (4-8 mm), large gravel (8-64 mm), cobble (64-256 mm), small boulders (256-512 mm) and large boulders (>512 mm) (Platts et al. 1983). c) Bottom oxygen, defined as oxygen level at the bottom of the deepest point in a pool. Measurements were made with a portable DO meter. d) Mean bottom water temperature, estimated by taking the mean of all bottom 12 / / East FO,k 48 N 46 44-45 8usr& Cr. 43 Cdr Cr. 40.41-42 39 37.38 36 5 35 34 .9, "04 31.32 30 29 27 Krt Figure 4: Distribution of deep pools (mean depth > 0.8 m) in Streamboat Creek, OR, sampled in this study. 13 a N 2 Ct C 3 4 9 Km 10 C) Figure 5: Distribution of deep pools (mean depth > 0.8 m) in Canton Creek, OR, sampled in this study. 14 temperature. Measures were made with a digital probe. e) Temperature coefficient of variation, estimated as the grand mean average temperature in the water column divided by its standard deviation. f) Area, defined as the sum of areas between successive transects. g) Mean depth, estimated by dividing the sum of depths at each transect point by n+1 to account for zero depth at the margins (Welch 1948). h) Volume, estimated by multiplying pool area by mean depth. i) Maximum depth, the maximum depth within the pool. j) Maximum length, defined as the length along the pooi axis that connected the points located in the middle of the channel that corresponded to the first and last transects respectively. k) Shore sinuosity index, computed as the total shoreline length of the pooi divided by twice the maximum length. A value of 1 implies a rectangular shape. I) Depth ratio, defined as mean depth/maximum depth (Carpenter 1983). m) Proportion of littoral area, defined as the proportion of sample points at each transect having a depth 0.5 m. n) Proportion of cover provided by ledges, defined as the percent of the perimeter of a pool occupied by overhanging ledges > 30 cm in width. o) Presence of waterfalls, defined as plunge at the head of the pool 1 m in height. The thermal gradient in Steamboat Creek was determined by measuring water temperature in the middle of the water column at a point every 1.6 km 15 between 3 and 5 pm at the beginning of September 1991 and 1992. Temperatures of selected tributaries were measured at the junction with Steamboat Creek. 2.3. Basis for selected variables Physical characteristics of habitat play an important role for salmonid distribution (Lanka and Hubert 1987; Scarnecchia and Bergersen 1987; Heggens 1988; Nelson et al. 1992). Several past studies have also used depth to assess pool quality (e.g. Duff and Cooper 1976; Parsons et al. 1982; Binns 1982; Oswood and Barber 1982; Helter et at. 1983; Plaffs et at. 1983; 1987). I considered depth a major variable because deeper pools are expected to provide refuge from predators (Skeesick 1989). Pool length was used in this study despite its not being considered by other researchers. I assumed that longer pools could provide better escapement from predators. Area and volume were considered as relevant morphometric variables because pools with larger areas and volumes could hold more individuals (Nickelson et al. 1979; Dunn 1981; Grette 1985; Faush and Northcote 1992). The depth ratio was also included in the analysis because it may represent the crossshape of a pool. This factor may be important for adult summer steelhead because it is representative of the general shape of the pool. Studies on bull-trout (Sa/velinus con fluentus) in Idaho for example, have shown that this species preferred pools with constrained sides provided by rock walls (A. Thurow, Intermountain Research Station, Boise, Idaho, corn. pers.), but no such information 16 is available for steefhead. Although studies in streams have used other related shape indices (see Olson-Rutz and Marlow 1992), such as width/depth ratio (Beschta and Platts 1986), such measurements would be appropriate only in very shallow channels and are not applicable for deep pools. I used shore sinuosity to represent shore habitat diversity; more convoluted shores indicate a greater array of different microhabitats in the pool and a larger land-water interaction. This latter factor could be important in order to predict potential sources of cold water provided by underground flows and seeps coming from upland side slopes. Substrate was considered another important variable that could influence the presence of fish in pools. Adult summer steelhead have a preference for certain substrate types (Hampton and Aceituno 1988). In addition, substrate may provide cover and refuge (Everest and Chapman 1971; Binns 1982; Freese 1982). Pool cover characteristics were also included in the analysis (Buttler and Hawthorne 1968; De Vore and White 1978; Mesick 1988). I considered two sources of cover: cover by overhanging ledges because it provides an available permanent refuge and cover by shade provided by the riparian forest. Finally, water temperature and dissolved oxygen were included because they influence metabolic processes of fish. The influence of temperature was considered by two parameters: a) the coefficient of variation, which is appropriate to summarize thermal heterogeneity and b) mean bottom temperature, which best represents the presence of cool spots and cool bottoms. 17 2.4. Definition of cool and warm pools Mean bottom water temperature was used to classify pools as either cool or warm. Mean bottom temperature is a better indicator of pool thermal characteristics than other parameters, such as mean water temperature, because it is less likely to be influenced by air temperature variation. The mean bottom temperature is also a suitable indicator of the volume affected by cool seeps or stratification processes. Previous studies considered pools as cool when there was at least 3 °C temperature difference between the pool and the mainstem in coastal streams in California (Ozaki 1988; Nielsen 1992). Matthews et al. (1994) defined stratified pools as those having a bottom-surface difference larger than 0.5 °C. In this study I used an approach based on physiological requirements. I considered 19° C to be the limit between cool and warm pools. I believe that 19 o is the upper limit of the preferred temperature of steelhead. The preferred temperature is a key eco-physiological concept which defines the range of temperature where most of organisms will congregate or spend most of their time in a thermal gradient (Reynolds 1977; Reynolds and Casterlin 1979). Table 1 lists a rather broad spectrum of preferred temperatures for rainbow trout. Most are for luvenile rainbow trout, and despite the wide temperature range, 19-20 °C seems to be the upper limit of the preferred temperatures. Since rainbow and steelhead are the same taxonomic species, I assumed that they would have similar physiological requirements. Even if some discrepancy has been noted between field and laboratory results 18 (see Reynolds 1977 and references therein), there has been a reasonably close correlation between laboratory and field data (Stauffer et at. 1980). A temperature of 19 °C is also consistent with field observations made by McMichael and Kaya (1991) who noted that rainbow trout strongly decreased in abundance in waters with temperatures above > 19 °C. In turn, Matthews et al. (1994) found that rainbow trout selected pool temperatures between 13 °C and 19 OC. The U.S. Environmental Protection Agency (1986) also recommended that summer water temperature should not exceed 19 °C in order to avoid physiological stress of fish. 2.5. Fish sampling Adult summer steelhead were counted by direct observation. One diver using a mask and snorkel moved back and forth across a pooi in an attempt to locate all fish. Pools were surveyed twice in August and early September of 1991 and 1992. Pools were defined as USED or NOT USEDbased on presence or absence of adult summer steelhead and not on abundance. Although both measures are related (Wright 1991), presence-absence was chosen for the following reasons: a) binary data provide ecologically meaningful information with lower field cost (Green 1979); b) presence-absence data emphasize the limits of environmental tolerance (Hinch et al. 1991) and would be preferable if variation in adult abundance were important in determining pool occupancy (Rahel 1990); C) density may not be 19 Table 1: Reported preferred water temperature for rainbow trout. L.N.A.: location not available. Stage Temperature preference (°C) Location Author Adult 13.0 laboratory Garside & Tait 1958 Adult 16.5 lake Michigan Spigarelli 1975 Adult 10.0-17.0 Adult 12.9-19.0 American R., CA Matthews et al. 1994 Adult 17.7-19.0 Pit A., CA Baltz et al. 1987 Juvenile 19.2-19.8 laboratory Cherry et al. 1977 n.a Coutant 1977 Juvenile 17.2 laboratory Hokansson 1977 Juvenile 18.0-19.0 laboratory McCauly & Pont 1971 Juvenile 13.6 laboratory Fergurson 1958 Juvenile 18.0 laboratory Cherry et al. 1975 Juveniles 10.0-13.0 n.a Bell 1986 Juvenile 18.0 n.a Schlesinger & Regier 1983 20 necessarily correlated with habitat quality (Van Home 1983) and d) some pools in Steamboat Creek were difficult to sample because of poor visibility and human disturbances. Fish were considered present in a pool if at least one adult summer steelhead was reported during two consecutive years. 2.6. Statistical analysis Odds ratio (Kahn and Sempos 1989) based on a 2x2 contingency table was used to measure the association between water temperature and use of deep pool. Specifically, my hypothesis was that cool pools were occupied to a greater extent by adult summer steelhead than were warm pools in August-September 1991 and 1992. Odds ratio is an alternative to traditional Chi-square statistics because it is not affected by number of observations and can be used as a directional test (Fleiss 1973). The null hypothesis was that the odds ratio of use of deep pools was independent of water temperature and therefore the odds ratio should be equal to one. Differences in pool types used by fish and their relationship to ecological characteristics were explored using a multivariate approach. The rational for such an approach is based on the fact that a suite of potentially related factors influence presence and abundance of species (Green 1978). Multivariate analysis captures the Hutchinson's n-multidimensional niche concept, thus not surprisingly, in recent years fish ecologists have shown a strong preference for multivariate methods, as documented by the extensive literature on habitat suitability models (e.g., Bovee 21 and Zuboy 1988) and habitat-standing crop models (see Faush et al. 1989 for a review). In this study I used discriminant analysis (DA) to determine which physical features were most strongly associated with presence of adult summer steelhead in pools in Steamboat Creek. This statistical method has proven useful in relating fish distribution and habitat use by others (e.g., Bowlby and Roff 1986; Baltz et al. 1987; Grossman and Freeman 1987; Layher et al. 1987; Doloff and Reeves 1990; Jowett 1990; Capone and Kushlan 1991; Larscheid and Hubert 1992; Nelson et al. 1992). Discriminant analysis has two goals (Williams 1983; Williams and Titus 1988). One is to predict the group to which an observation belongs based on measured variables; this procedure is called predictive discriminant analysis and uses a discriminant function. A second purpose of DA is to exhibit an optimal separation of groups. This approach, called descriptive discriminant analysis, is associated with a linear function referred to as canonical variate or canonical function. Descriptive analysis is a valuable method to for identifying a relatively small set of variables that maximizes the difference among-groups. In this study, both methods were applied. To select potential discriminant variables, several steps were followed. First, potential multicolinearity was reduced by eliminating highly correlated variables (r> 0.80). Second, a stepwise regression method (Proc stepdisc, SAS 1985) was used but only as a first guideline. The use of stepwise regression is a controversial process (Pimentel 1979). Differences in relationships among variables can yield predictors that do not reflect population differences (Tabaschnick and Fidell 1989). Although stepwise regression can produce a set of optimal discriminating variables, it cannot guarantee that this set is the best combination (Klecka 1980). According to Legendre and Legendre (1983), stepwise regression must be used to select only the most important predictors from a very large number of descriptors. Performance of discriminant analysis can be improved by examination of each possible variable, as recommended by Cochran (1964) and McCabe (1975). Thus, as a third step, a separate analysis was done by performing an univariate analysis and observing the squared canonical correlation and number of entities correctly classified. Those variables with the largest squared canonical correlations were then combined sequentially to find which maximized the canonical correlation and the number of pools correctly classified. Canonical scores were tested for normality by examining their skewness and kurtosis. Equality of variance-covariance matrix was checked by a Chi-square test (Morrison 1967). Wilks lambda statistics was used to test differences between centroids. To interpret the discriminant function, structure pattern (loadings) and standarized coefficients were used. Structure pattern gives more substantive interpretation than standarized coefficients, although these values are sometimes useful to indicate redundancy among variables (Klecka 1980; Stevens 1986). A problem typically encountered in DA is unequal group sample size. To evaluate classification accuracy the Cohen's Kappa statistic (Cohen 1960; Titus et 23 al. 1984) was used. This statistic is used to express the proportion of pools correctly classified by the discriminant function after the effect of correct classification by chance is removed. Predictive classification was achieved by two avenues. One attempt was performed applying a classification criterion determined by a generalized squared distance (Proc discrim SAS 1985). This criterion put each observation in the class from which it has the smallest generalized squared distance. A second procedure consisted of developing a non-parametric discriminant analysis, which has the form of a dichotomous key (KendaN and Stuart 1966; Kendall 1980) (see Fig. A.2). The method is not restricted by statistical assumptions of parametric statistics (Seber 1984). Use of discriminant methods in the form of a field key has been a successful way to simpflfy the interpretation of the data and provide a suitable field tool for managers and biologists unfamiliar with multivariate analysis (Tonn et al. 1983). To test the efficiency of the discriminant models outside Steamboat Creek, pools in Canton Creek were used as a validation. As noted by several authors (e.g., Reckhow et al. 1987), when the data that are used to develop the classification procedures are also used to estimate the accuracy of prediction, true classification error rate is underestimated. Ideally, discriminant analysis should be completed by checking the performance of the model with a different data set (Picard and Berk 1990; Beauchamp et al. 1992). Other general statistical procedures included the use of the Wilcoxon paired test (Zar 1984) to compare fish abundance in deep pools between different years 24 (August-early September 1991 and 1992), and correlation coefficients to analyze the association between environmental variables in pools. In turn, differences in morphometric parameters and mean bottom water temperature between cool and warm pools were examined by applying the normal approximation of the MannWhitney test (Zar 1984). Environmental variables between poois in Canton and Steamboat streams were compared using a t-test followed by a power analysis according to Cohen (1988). 25 Ill. RESULTS 3.1. Steamboat Creek thermal regime Steamboat Creek had a similar thermal gradient in the summers of 1991 and 1992 (Fig. 6). Water temperature ranged from 15 °C to almost 22 °C . Only the upper part of the stream and a short segment located in the middle course exhibited a temperature well below 19 °C. Three segments were identified along the Steamboat Creek based on thermal characteristics: a) from km 0 (Umpqua River) to km 17, where water temperatures decreases slightly in the last 5 km; b) from km 17 to km 20, where temperatures increased sharply and; c) from km 20 to km 32, where temperatures decreased (Fig. 6). The influence of Big Bend Creek (km 17) made a thermal division in Steamboat Creek (Dambacher 1991). Other cold tributaries such as Steelhead Creek, Reynolds Creek and Cedar Creek did not have a major impact on thermal profile of Steamboat Creek. At km 25, the temperature of Steamboat Creek sharply decreases becauser of the effect of the riparian vegetation. My results were consistent with Brown et al. (1971) and 1-loladay (1992) and agree with extreme values reported by Reeves et al. (1987). Based on these considerations, I concluded that water temperatures observed in 1991 and 1992 represented an average summer profile of Steamboat Creek. 26 fin 1) t) p H LU 4.5 7.7 10.4 13.9 17.0 20.5 23.8 27.0 30.2 Distance (km) September 3/91 A-- September 2/92 Figure 6: Thermal profile of Steamboat Creek. Zero point corresponds to the junction with the North Umpqua River. Arrows indicate main tributary junctions. CA: Canton Creek; S: Steelhead Creek; R: Reynolds Creek; BB: Big Bend Creek; C: City Creek. Temperature differences between tributaries and Steamboat Creek increased in a downstream direction (Table 2). Water temperatures of tributaries were generally lower than those in Steamboat Creek in 1991 and 1992. 27 Table 2: Water temperature (°C) of Steamboat Creek tributaries. Numbers in parenthesis represent the temperature in Steamboat Creek above the junction with the respective tributary. Temperatures were measured between 1 and 4 p.m. Tributaries are ordered in an upstream-downstream position. Tributary August 7, 1991 September 8, 1992 Horseheaven 16.7 16.0 (17.1) City 15.7 17.5 (18.7) Little Rock 19.1 20.2 (18.7) Long 13.7 14.8 Buster 15.0 (21.5) Cedar 16.9 (20.8) 16.7 (21.2) Big Bend 13.1 (21.2) 13.6 (20.0) Johnson Reynolds Steelhead 16.7 (17.3) 17.4 14.5 (17) Siwash Spring Grass Canton 17.1 (17.3) 15.7 (18.5) 15.3 13.5 16.3 17.5 20.6 (19.9) 3.2. Distribution of deep pools There were 0.83 deep pool/km in Steamboat Creek. Results of the systematic sampling showed that deep pools were only 4 % of total number of habitat units Deep pools were distributed in two main areas. One area was from the junction with the North Umpqua River to near Cedar Creek (Fig. 4). This segment contained 84 % of the deep pools. This area in turn, can be divided in two segments: a) a lower segment from Canton Creek to Steelhead Creek, with a pool density of 1.33 pool/km. This segment included the Black Gorge (from pools 15-15 to Steelhead Creek); b) an upper segment from Steelhead Creek to Cedar Creek. In the latter segment, pool density was 0.62 pools/km. Most of the deep pools were found clustered between Long Creek and City Creek (Fig.4). Upstream of City Creek Steamboat Creek no deep pools were found. 3.3. Pool characteristics 3.3.1. Morphometry Main statistical parameters such as the mean, the standard desviation and range of measured variables in Steamboat Creek are portrayed in Table 3. 29 Table 3: Statistical parameters of measured variables in study pools. Variable Ledge cover Bank riparian forest Sand Bedrock Small boulders Large boulders Small gravel Large gravel Area (m2) Volume (m3) Mean depth (m) Maximum depth (m) Depth ratio Mean width (m) Littoral area proportion Maximum length (m) Shore sinuosity index Bottom oxygen (mg.02.L1) Water temperature coefficient of variation Mean bottom water temperature (°C) Mean 0.31 0.37 0.06 0.45 0.16 0.14 0.06 0.05 565 856 1.57 3.5 0.50 13 Standard desviation 0.39 0.29 0.09 0.20 0.09 0.14 0.06 0.04 389 684 0.47 3.15 0.50 14.2 Range - 0.95 0.75 0.37 - 0.81 - 0 0 0 0 0 0 0 0.33 0.63 0.21 - - 0.17 114- 1431 97 3035 3.46 5.00 - 0.77 - 24.40 - 0.47 11 77 1.08 2.64 0.86 1.75 0.30 4.85 0.12 0.25 30 1.40 8.75 0.29 37.4 0.34 1.91 1.5 1.70 1.66 0 19.69 1.95 - - - 12 5.44 16.59 Scour pools (Bisson et al. 1982) were the most common type (85%) of 22.68 p001 observed. Trench pools comprised 5% of the total pools, and plunge pools formed by waterfalls represented 8% of the total. Plunge, dammed or backwater pools formed by woody debris were not observed in either Steamboat Creek or Canton Creek. Results of the correlation analysis among the environmental variables are 30 shown in Table 4. Few variables showed a high correlation > 0.70. Mean bottom pool temperature showed no statistical relation with morphometric parameters such as area (r= -0.04; P< 0.50); volume (r= -0.02; P<0.50); mean depth (r= -0.12; P<0.20); maximum depth (-0.06; P<0.20); depth ratio (r= -0.18; P<0.50); mean width (r= -0.08; P<0.20) and maximum length (r= -0.01; P<0.50). 3.3.2. Water temperature Of the 38 deep pools surveyed, 39 % were classified cool pools. Warm pools in Steamboat Creek were most abundant in the first 12 km, but also they were located in the middle and upper part of Steamboat Creek. In turn, no cool pools were detected in the lower segment. The uppest segment of Steamboat Creek did not have deep pools (Fig. 7). Mean bottom temperature was 17.7 °C and 20.9 °C in cool and warm pools, respectively and this difference was significant (Z=5.13; P0.001). Cool and warm pools did not differ in area (Z= 0.44; P0.69), volume (Z= 0.24; P> 0.81); mean depth (Z= -1.31; P0.19); maximum depth (Z= -0.91; 0.36), mean depth/maximum depth ratio (Z= -0.36; P> 0.72), mean width (Z= 0.22; P 0.82) and maximum length (Z= 0.07; P0.94). Table 4: Correlation matrix among physical features of deep pools in Steamboat Creek, OR, summer 1991-1992. LED: ledge cover proportion; S:sand proportion; BR: bedrock proportion; LB: large boulder proportion; C:cobble proportion; SB: small boulder proportion; SG: small gravel proportion; LG:large gravel proportion; A: Area; VOL: volume; MD: mean depth; MAD: maximum depth; DR: depth ratio; MW mean width; LIT: littoral area proportion; ML: maximum length; SSI: shore sinuosity index; BO: bottom oxygen; TVC: temperature variation coefficient; BT: mean bottom pool temperature (see text for further explanation). IC RF S DR LB C SB SC IG A P_c 1.00 RI' 0.13 1.00 S 0.04 -0.08 1.00 BR -0.06 0.15 -0.40 1.00 LB 0.05 -0.35 0.18 -0.60 c 0.25 0.49 0.00 -0.37 0.34 1.00 SB 0.13 0.04 -0.15 -0.37 -0.08 0.26 1.00 SO -0.33 0.00 -0.07 -0.30 -0.16 0.24 -0.05 10 -0.10 -0.05 -0.29 -0.13 -0.18 0.23 -0.03 0.38 1.00 A 0.59 0.40 0.05 0.05 -0.31 0.55 0.02 -0.08 0.08 1.00 0.01 0.90 VOL ZP4 ZX DR P4W LII Ml SI DO Br 1.00 1.00 VOL 0.64 0.36 0.02 0.00 -0.17 0.44 0.09 -0.17 ZM 0.66 0.07 0.07 -0.14 0.16 0.14 0.04 -0.22 0.04 0.44 0.67 1.00 2X -0.24 -0.37 -0.18 0.01 0.17 -0.32 0.22 -0.14 -0.12 -0.55 -0.41 0.71 1.00 OR 0.41 -0.16 -0.10 -0.13 0.25 -0.11 0.26 -0.29 0.00 -0.02 0.27 0.70 0.48 1.00 MW 0.38 0.45 0.02 -0.07 -0.40 0.55 0.23 0.12 0.25 0.78 0.66 0.25 -0.40 -0.05 1.00 -0.06 0.10 0.00 0.28 -0.39 0.04 -0.01 0.01 -0.07 0.16 -0.10 -0.34 -0.44 -0.57 0.22 1.00 141 0.55 0.24 0.06 0.08 -0.09 0.32 -0.11 -0.20 -0.07 0.87 0.82 0.48 -0.51 0.02 0.42 0.11 1.00 SI 0.10 -0.09 -0.13 -0.14 0.15 -0.16 0.35 -0.11 0.00 -0.28 -0.07 0.36 0.41 0.67 -0.03 -0.39 -0.38 1.00 00 -0.33 -0.23 -0.09 -0.28 0.07 0.19 0.28 0.12 0.26 -0.23 -0.28 -0.25 0.17 -0.06 -0.22 0.00 -0.14 -0.03 1.00 LII TVC 1.00 IVC 0.18 0.22 0.04 0.26 -0.22 0.09 -0.05 -0.22 -0.19 0.25 0.30 0.20 -0.05 0.07 0.21 -0.12 0.16 -0.13 -0.43 1.00 81 017 -0.28 0.73 -0.23 0.17 -0.06 0.12 0.02 -0.25 -0.04 -0.02 -0.12 -0.06 -0.18 -0.08 0.05 -0.01 -0.14 -0.09 -0.19 1OO -s 32 ', A 0 0 C 1) C C o 2.5 5 7.5 10 12.5 15 17.5 20 22.5 25 27.5 30 32.5 Distance (km) Figure 7: Distribution of cool pools ( 19°C mean bottom water temperature) and warm pools (> 19°C mean bottom water temperature) in Steamboat Creek. Pools of similar category that were located close together are shown as only one point. Zero point corresponds to the junction with the North Umpqua River. 3.4. Fish abundance and distribution 3.4.1. Systematic sampling Adult steelhead were found in deep pools. Almost no fish were observed in shallow pools or other habitats such as glides and riffles. Cool tributary junctions such as Johnson Creek, Steelhead Creek and Big Bend Creek, were all shaHow and did not contain fish. Although I did not survey the tributaries, past studies suggest that these tributaries, did not hold fish because of low summer flows (Wroble and Roelof 1985). Two shallow pools (0.40-0.60 m mean depth) harbored one fish during the summer of 1991, but not in 1992. These pools, according to previous definitions (see 2.5), were not considered in the analysis. 3.4.2. Pool sampling Number of cool pools occupied in August-early September 1991 and 1992 surpassed the number of warm pools occupied by adult summer steelhead (Table 5). However not all cool pools were contained adult summer steelhead. More than 50 % of deep pools remained unoccupied in both years (Table 6). Table 5: Total number of adult summer steelhead in cool and warm deep pools in Steamboat Creek during August-early September 1991 and 1992. (S.D: standard desviation). Year Warm pools Cool pools Total Mean S.D 1991 2 238 240 8.57 22.37 1992 6 229 235 6.03 16.74 34 Table 6: Percent of cool pools occupied by adult summer steelhead in Steamboat adultsand mean density (fish/rn3) Creek, percent of cool pools having in cool pools (6,), warm pools (6w) arid all deep pools (6) Year % occupied % cool pools cool pools with 5 fish C 6W 1991 43 5 0.018 0.002 0.008 1992 34 5 0.017 0.0005 0.007 There was no significative difference in the mean number of adult summer steelhead in pools in Steamboat Creek in August-September 1991 and August- September1992 (Wilcoxon paired-test: T= 20.5 > =13; P>0.10). This result suggests that abundance in each pool was similar between years. Most of the adult summer steelhead were found in the middle part of Steamboat Creek, but some adults were also detected in the upper segement. Three pools located downstream from Big Bend Creek contained 72 % of the total fish number in 1991 and 60 % in 1992 (Fig. 8). 35 [00- 80- 60- 1991 40 E I..) H 1992 40- 60- 80I 11 13 12 15 14 I I I I I I I I I I I I I I I I I I I I 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 Pool No Figure 8: Mean number of adult summer steelhead in deep pools in Steamboat Creek during August-September 1991 and August-September 1992 (see figure 4 for pool locations). Note: pools 1-10 are located in Canton Creek (see Fig. 3 for pool locations). The largest number of fish was observed in pools 33 and 36 in both years. Pool 36 is located immediately downstream from the junction with Big Bend Creek. The importance of this pool been already noted (Dambacher 1991). As was 36 previously pointed out, Big Bend Creek strongly influences water temperature of Steamboat Creek. Very close to pool 36, pool 35 also had adult summer steelhead. However, its small volume was probably a Hmiting factor. Big Bend Creek affects Steamboat Creek for several kilometers downstream. Meanwhile pool 34 harbored few adult of summer steelhead, but pool 33 had a noticeable number of adults. In general, I found that adult abundance in those pools agreed with previous historical records (Roelof unpublished data). On the other hand, a moderate number of adults were noted in pool 48. The number of adults in this pool even surpassed the number of adults noted in pool 42. This pool has been considered as the final deep pool in Steamboat Creek by previous surveys and usually harbored a moderate number of adult summer steelhead (Roelof unpublished data). However, results of my study showed that pool 48 must be considered as the last deep pool in the system that harbors adult summer steelhead. Furthermore, pool 48 had a higher number of adult summer steelhead than pool 42 according to past records. 3.5. Temperature-abundance relationship Fish abundance decreased with increasing mean bottom temperature in 1991 and 1992. As shown in Figure 9, a clear pattern was observed: Adult summer steelhead numbers were variable in those pools never had high numbers of fish. 19 °C, but pools > 19 0C 37 ci) -Q E U- 16 17 18 19 20 21 22 23 Pool mean bottom temperature (C) 1991+ 1992 Figure 9: Relationship between mean water temperature at the bottom of the pool and number of adult summer steelhead in study pools in Steamboat Creek in August-September 1991 and 1992. Odds ratio results showed that during the summer of 1991, the estimated odds that a cool pool was used by an adult (Q) was 1.66 (10/6). In turn, the odds that a warm pool was used (O,) was estimated as 0.20 (2/10). As a measure of association between the presence of cool pools and their use, the odds ratio OR (O/O) was estimated as 8.33 (P <.007). For the summer of 1992 Q and O were 1.28 and 0.1 respectively and the OR was 12.85 (P'zO.004) (Table 7). Table 7: Results of the odds ratio test of pools with different mean bottom water temperature that were used and not used pools by adult summer steelhead in Steamboat Creek, OR, in August-September 1991 (A) and August-September 1992 (B). See text for further details. A USED COOL 10 WARM TOTAL NOT USED TOTAL 6 16 2 10 12 12 16 28 ODDS RATIO 8.33; Z = 2.43; P<0.007 B USED NOT USED TOTAL COOL 9 7 16 WARM 2 20 22 TOTAL 11 27 38 ODDS RATIO = 12.85; Z = 2.60; P<0.004 The Woolf test for interaction was applied to examine if both years could be combined. A Chi-squared value of 0.13 (P<0.001) permitted the data set to be merged and to estimate a common odds ratio of 11.35 (P <0.007). The result was that the odds of cool deep pools being used by adult summer steelhead were estimated to be 11 times as large as the odds of the use of warm pools. 39 3.6. Relationship between pool environmental characteristics and pool use 3.6.1. Selection of predictive variables No single variable was statistically significant as a predictor for discriminating deep pool used or not used by adult summer steelhead in Steamboat Creek. Square canonical correlations (SCC) derived from univariate analysis were highest for mean bottom water temperature (SCC= 0.33), mean depth (SCC= 0.30) and volume (SCC= 0.25). Use of an iterative solution for variable selection, produces a parsimonious five variable solution. In other words, with five variables I found the minimum number of variables that accounted for the largest proportion of explained variance. Bank riparian forest, proportion of large boulder, mean depth, mean bottom pool temperature and maximum length were identified as the best subset of discriminating variables. Such variables differ between both the USED and NOT USED pools (Table 8). If a smaller subset of variables would be used, some amount of explained variance must be sacrificed. For instance, if mean bottom pool temperature, maximum length and mean depth would be the only used variables, only 62 % of the variation between the groups would be explained. 40 Table 8: Mean, standard deviation (S.D) and range of variables discriminating between USED and NOT USED pools by adult summer steelhead in Steamboat Creek, OR. August-September 1991 and 1992. RF: bank riparian forest; LB: proportion of large boulder proportion; MD: mean depth (m); ML: maximum length (m); BT: mean pool bottom temperature (°C). NOT USED USED Mean S.D RF LB MD 0.50 0.16 ML BT 42.22 0.22 0.15 0.57 18.72 1.77 1.71 18.71 Range 0-0.75 0-0.63 1.25-3.46 11-77 16.6-21.2 Mean S.D Range 0.28 0.12 0.27 1.32 0.35 32.20 20.45 14.52 1.44 0.60 - 0.39 0.86-2.38 11-67 18.0-22.6 0.11 0 0 - Wilkes Lambda was 0.30 (P>F 0.0001), which indicated that a significant differences between groups (centroicis) was present relative to the amount of dispersion within the groups. No significative differences were found for equality of variance-covariance matrices between groups (Chi-square= 20.48; P<0.1). Adjusted square canonical correlation was 0.82. In other words, 69 % of the variation in the canonical function was explained by differences in means groups. This value is a multiple correlation coefficient that represents the amount of the canonical variation and is a measure of the discriminant performance. Diagnosis of skewness and kurtosis performed on canonical scores, showed that the NOT USED group had a normal distribution (skewness = -0.64; kurtosis = 0.37), whereas the USED group showed a greater departure although not serious enough to invalidate the analysis (skewness = -0.01; kurtosis = 0.49). 41 Analysis of structural canonical patterns and standarized coefficients showed that mean depth, maximum length, bank riparian forest had positive values, while mean bottom water temperature correlated negatively (Tables 9 and 10). In bath cases the relative weight of the variables were similar: mean bottom water temperature and mean depth were the most significative variables as shown by the largest structure and standarized coefficients. Table 9: Standarized canonical coefficients derived from the discriminant analysis. Variable Bank riparian forest Large boulder proportion Mean depth Maximum length Mean bottom water temperature Standarized coefficient 0.49 0.52 0.66 0.56 -1.09 Table 10: Structural patterns derived from the discriminant analysis Variable Bank riparian forest Large boulder proportion Mean depth Maximum length mean bottom water temperature Structure pattern 0.45 0.32 0.66 0.54 -0.69 42 Proportion of large boulders appear to have a small influence, but removal of this variable from the analysis, reduced the square correlation coefficient from 0.69 to 0.64. The canonical function defined a gradient from long, shady, deep pools with a coarse substrate and cooler bottoms (USED group) to pools that were shorter, sunnier, shallower, with more fine particulate substrate, homogeneous in temperature and with warmer bottom temperature (NOT USED group). Plot of the canonical scores is presented in Figure 10. The NOT USED group had a wider distributions and overlapped slightly with some pools from the USED group. The obtained classification has a hit-ratio of 0.89 (34/38). A % of the USED pools were correctly classified, whereas only a % of NOT USED pools were incorrectly classified. After using the jackknife resampling procedure, only 4 samples were incorrectly classified (Table 11). Application of the Kappa statistics showed that classification was 86% better than derived from random assignment (Z= 4.77; P<0.001). This result was highly significative since priors possibilities differed between the USED and NOT USED groups. 43 Warm Shallow Bottom temperature Depth Small Substrate Scarce Shadow Little _ ' Length Cold Deep Coarse Abundant Much Figure 10: Plot of discriminant scores based on analysis in Steamboat Creek. Vertical lines on the block and the circle respectively indicate the centroid position. Horizontal line at the extremes corresponds to the ranges. 44 Table 11: Number of pools in Steamboat Creek correctly classified by discriminate analysis after jacknife resampling procedure. NOT USED NOT USED USED TOTAL 22 2 24 USED 2 12 14 TOTAL 24 16 38 PRIORS(%) 63 37 Since group sizes were unequal, these results may indicate a stable classification. It should be noted that because 24 pools belong to one group (NOT USED), it would be possible get a hit ratio of 0.63 (24/38) by simply assigning all the pools to the NOT USED group and yet obtain a significativ classification due to chance (Morrison 1969; Hair et al. 1983). 3.7. Pool use prediction 3.7.1. Square generalized distance This procedure showed that for Canton Creek pools, a hit rate of 0.7 was obtained (Table 12). All NOT USED pools were correctly classified but 3 of 5 USED pools were classified as NOT USED pools. Use of the Kappa statistics indicated an improvement over chance assignment of 40 % (Z= 1.26; P<0.10). 45 Table 12: Number of pools in Canton Creek correctly classified by discriminant analysis. NOT USED USED TOTAL NOT USED 5 0 5 USED 3 2 5 TOTAL 8 2 10 50 50 50 PRIORS(%) 3.7.2. Development f fjid y Discriminant analysis is a powerful technique to separate used from not used deep pools based on major ecological variables, but it is sometimes difficult to use directly in the field. Biologists wanting to predict the use of a deep pool based on its characteristics, could apply DA developed in Steamboat Creek. This step involves a computational procedure. Development of a field key is an alternative for evaluating the potential of a pool to be used by adult summer steelhead. To develop a key, two important conditions must be met: it should be based on variables that can be easily measured in the field, and at the same time, the variables need to be ecologically meaningful. Following the variable selection in the DA, I used maximum length, bank riparian forest and mean bottom water temperature since they performed well in the univariate discriminant analysis. 46 Presence of waterfall (a binary variable) was also included, since 20% of the pools in Canton Creek had waterfalls (i.e., 1 m high). Mean depth was replaced by maximum depth to facilitate the key use. The deepest spot in the pool can be used as surrogate it a significant relationship exists between mean depth and maximum depth (see table 3). In turn, proportion of large boulders was removed to facilitate the key use. This parameter is difficult to measure from the bank. One problem noted in the development of the key was the presence of outliers". For instance, for the variable bank riparian forest" (RF), there was one USED pool with a value of 0, that definitely departed from the rest of the observations. In the case of the maximum length (ML), I found a USED pool as short as the shortest NOT USED pool. Obviously, putting the cut-off point at FC =0 or ML= 11 would yield no possible discrimination, when in fact these cases are rather isolated. After ranking mean bottom water temperature and maximum depth, the following dichotomous key was derived from Steamboat Creek pools: 1. Maximum depth < 2.5 m .........................NOT USED 1'.Maximum depth 2.5 m ......................... 2 2. Bank riparian forest < 20% ....................... NOT USED 2'. Bank riparian forest 3. Maximum length 20 % .................... 3 25 m ..........................NOT USED 3'.Maximum length > 25 m ........................... 4 4. Bottom temperature 19°C ..................... USED 47 4'.Bottom temperature > 19°C. 5 5. Pools with waterfalls .................................... USED 5'.Pools without waterfalls............................... NOT USED The key correctly identified 4 of the 5 USED pools and all the NOT USED pools in Canton Creek. The misclassified pool was a short rounded pool with a waterfall. IV. DISCUSSION 4.1. Thermal characteristics of the system Steamboat Creek is a complex thermal system that exhibits a strong variation in water temperature along its course. Big Bend Creek exerted a strong influence in Steamboat Creek. Brown et al. (1971) emphasized the importance of this tributary because of its relatively large flow and low water temperature. Although other tributaries were colder than Steamboat Creek, their effect on temperature of the main stem is negligible because of the relatively small amount of water in them (Brown 1971). Local topographic and geological factors can also influence thermal patterns (Smith and Lavis 1975). Bjornn et al. (1968) noted that thermal changes can take place in very short distances. In contrast, Canton Creek had a uniform profile where temperature surpassed the optimum conditions for adult steelhead in the middle and lower course. Past logging activities and road building have strongly reduced the riparian vegetation and increased water temperature. Since riparian vegetation is an effective control for solar energy (Beschta et al. 1987; Brown and Krygier 1970), direct solar radiation input may be a major factor causing water temperature increases in this stream (Brown and Krygier 1967; Moring 1975; Rishel et al. 1982; McGurck 1989; Heller et al. 1983; Holtby 1988). Mean bottom water temperature of individual pools was not associated with morphometric characteristics. For example depth has been generally linked with existence of cool bottoms, but Ozaki (1988) found that temperature differences between a pool and the mainstem were not related to mean depth, maximum depth, or area. This relation was also verified in Steamboat Creek, suggesting that even pools of moderate depth could be thermally stratified. Consequently variables such as depth, area or volume can not be used as potential indicators of thermal conditions in pools and therefore cannot be applied as an indirect method to predict presence of adult steelhead in deep pools. 4.2. Fish distribution patterns The use of deep pools by adult summer steelhead in Steamboat Creek was consistent with results from other studies of pools used by adult steelhead. Campbell and Neurier (1985) observed that in four western Washington Cascade streams that rainbow trout selected deep pools over other habitats. Jones (1979) found that only 7 % of adult summer steelhead in the upper Middle Fork Eel River held in pools < 1.5 m maximum deep. This percentage was even lower (3%) in the lower part of the Middle Fork Eel River (Jones 1992). Use of deep pools seems also to be a general pattern in other California streams (Higgins and Kier 1992). Nakamoto (1994) found that most pools used by summer steelhead in the New River, California were > 1 m mean depth. These results agree with the pattern found in the present study: adult summer steelhead in Steamboat Creek were rarely found in shallow pools (<0.8 m mean depth) and therefore researchers should use caution in estimating habitat suitability without considering habitat availability. 50 4.2.1. jj Qt cool warm Qools Water temperature influenced the distribution of adult summer stee)head and quantity and quality of available habitat in Steamboat Creek. Cool pools with adult summer steelhead average nearly 3 °C less than warm pools without adult summer steelheacj. This differed from results of Nakamoto (1994), who showed that cool pools holding summer steelhead in the New River, California, exhibited an average temperature slightly lower than pools without fish. Bowiby and lmhof (1989) proposed maximum temperature as the most important water quality variable for modeling trout habitat quality. Baltz et al. (1987) observed that temperature was an important factor in microhabitat choice and claimed that this factor proved to be a good predictor of species location. Freese (1982) suggested that water temperature should influence distribution of adult summer steelhead in the New River, California. In the John Day River, Oregon, Adams et al. (1990) demonstrated an inverse relationship between water temperature and steelhead abundance. Berman and Quinn (1991) also found that spring chinook salmon were associated with cold water sources in the Yakima River. Although adult summer steelhead in Steamboat Creek clustered in few pools cool pools, fish numbers were associated with mean bottom water temperature. Adult summer steelhead varied in number in those pools 19 °C, but they were never abundant in warmer pools. Such results support the argument that physiological constraints are appropriate to separate cool from warm pools. 51 Through results of this study, I concluded that cool pools were used more than warm pools as shown by odds ratio analysis. Since water temperature was not related with morphological factors, it must be considered as a major driving factor. In other words, fish used cooler pools because of the more suitable temperature and not because they were deep. However, fish showed an uneven distribution in cool pools. Some cool pools were never occupied and few warm pools had fish. I noted that most used warm pools had waterfalls. The effect of waterfalls may be associated with more oxygen in the water (oxygen solubility is inversely proportional to water temperature), or with natural barriers for fish movements. 4.3. Discriminant analysis In this study, discriminant analysis provided a suitable method for identifying variables most contributed to separating pools based on characteristics that ultimately defined the habitat quality. Other analytical procedures have dealt with habitat prediction of habitat use, but they are difficult to apply in the present study. For example, a common avenue has been to construct habitat suitabllfty curves (Bovee and Cochrtauer 1977) from which a composite habitat suitability index can be derived. However, in the case of rainbow trout and steelhead most of these studies were focused on juveniles stages (Bovee 1978; Shepard and Johnson 1985; Irvine et al. 1987; Waite and Barnhart 1992), and less frequently on adults (Raleigh et aL 1984; Baltz et al. 1991). Other alternatives such as the habitat quality 52 index (Binns and Eisermann 1979) required several fixed variables. Whereas both approaches are appropriate to determine habitat evaluation for reaches, segments, or overall general stream conditions, they are inapplicable for specific habitats such as deep pools. The good pertormance of discriminant analysis in this study resulted because assumptions associated with the method were not violated. Williams (1983) pointed out that discriminant analysis permits moderate departures from the assumptions, without affecting classification results. A key assumption of discriminant analysis is that group dispersions (variance-covariance matrices) should be homogeneous (Klecka 1980), and this condition was met in this study. Mutivariate normality is another important restriction. Strong lack of multinormality affects the computation of tests of significance and probabilities of group memberships (Klecka 1980). Fortunately no serious departures were noted with the pool data. Sample size also affects the accuracy of discriminant analysis. Williams and Titus (1988) recommended that the sample size for each group used in discriminant analysis should be equal or greater than three times the number of variables used. In the present study if five variables were used, a minimum of 15 pools were included in each group. The NOT USED group largely exceeded the minimum pool sample size, whereas the USED group would needed one additional observation. Finally, discriminant analysis requires that no singularities may be present. Highly correlated variables are not allowed in discriminant analysis; this makes sense intuitively since variables highly correlated are redundant and do not 53 contain new information. As shown before, few variables were highly correlated. Variable selection showed that mean bottom water temperature emerged as the main factor that accounted for pool group separation, corroborating the association found between temperature and pool use in the odds ratio analysis. However, high structure values for selected morphometric variables could indicate that pool occupancy is a sum of different factors and cannot be simplified only by looking at thermal characteristics. Some of the morphometric variables selected in the discriminant analysis have been identified previously as important features of pools used by fish (Duff and Cooper 1976; Parsons et at. 1982; Binns 1982; Oswood and Barber 1982; Heller et al. 1983; Platts et al. 1983; 1987). Jones (1982) reviewed steelhead distribution in California streams and found that populations appear to prefer deep, clear and cool pools. Lewis (1969) considered cover, volume, area and depth as relevant factors in explaining trout density and similar results were obtained by Rinne (1978). In the same vein, Heggens (1988) reviewing physical habitat selection by brown trout, pointed out that water depth, water velocity, streambed substrate and cover were the most important characteristics. For rainbow trout, large and deep pools with more than 30 % of the bottom obscured by factors such as depth, vegetation and boulders have been classified as those with the highest suitability index (Raleigh et at. 1984). Most of the identified features of pools used by adult summer steelhead in Steamboat Creek, can probably be associated with cover, which has been related to trout density (Stewart 1970; Fraley and Graham 1981), standing crop 54 (Harshbarger and Bhattacharya 1981; Wesche et at. 1987), population stability (Elser 1968; Lewis 1969) or predator avoidance (Mesick 1988). As noted by Bjornn and Reiser (1992), salmonids, like summer steelhead that enter freshwater streams some months before spawning, require appropriate cover to avoid disturbance and predation. Cover provided by riparian vegetation has been identified as a main source of overhead shade (Boussu 1954; Gibson and Keenleyside 1966; Buttler and Hawthorne 1968; Baldes and Vincent 1969; Lewis 1969; Harshbarger and Bhattacharya 1981; Freese 1982; Wesche et at. 1985). Also, shaded waters, may provide protection from predators (Helfman 1981). Advantages of streamside vegetation can be extended also to thermal effects. Bilby and Wasserman (1989) stated that 50-70 % of riparian conifer forest would be necessary to provide thermal refugia for fish. Physical dimensions of pools, as represented by length and depth, also emerged as an important characteristic of pools used by adult summer steelhead in Steamboat Creek. Past studies have addressed the importance of depth (e.g. Raleigh et al. 1984), but length may also be relevant. It can provide an additional source of escape from predators by allowing adult to swim back and forth. In the Steamboat Creek basin, adult summer steelhead selected some deep pools that were not very deep (1.25 1.75 m mean depth) but that were relatively long (e.g., pools 30, 33 and 42). Large boulders are an important component of pool substrate and may provide shelter for trout by forming protected areas, caves and crevices (Binns 55 1982; Freese 1982; Nakamoto 1994). De Vore and White (1978) found that brown trout used cover provided by artificial structures that were placed close to the bed stream. Nakamoto (1994) indicated that lateral scour pools with boulders were heavily used in the New River, California by adult summer steelhead. In the John Day river basin, Oregon, bull trout showed a preference for deep pools with boulder-rubble substrate in the summer (Skeesick 1989). Dambacher (1991) noted that the abundance of age >1 + steelhead was associated with large boulders in Steamboat Creek. Baltz et al. (1991) determined that 40 % of juvenile and adult rainbow trout in Rock Creek, California, were associated with boulders during the summer. 4.4. Validation of the discriminant model The use of parametric discriminant analysis appears to be a suitable method to identify physical features of deep pools used by adult summer steelhead in Steamboat Creek. However, its application in Canton Creek was only moderately successful. The classification was only slightly better than if was done by chance alone. Sampling size was probably a major reason for this result. Canton Creek contained only 10 deep pools and with such small sample size, natural variability produced by potential factors other than those considered in this study may be difficult to control. Also, Canton Creek is a 4th order stream, which carries approximately half of the water volume of Steamboat Creek. This may imply different bedforms and geomorphic characteristic (Platts 1979). Although the test 56 for difference between geomorphic characteristic between deep pools of Canton and Steamboat did not show significative statistical differences for most of study variables, conclusive results are not possible due to the low power observed in the t-test. 4.5. Effect of population size One potential limitation in this study is the influence of adult summer steelhead density on pool occupancy. It is reasonable to assume that pools may have some carrying capacity and agonistic behavior among adult summer steelhead may affect pooi use. This feature may be evident if adult summer steelhead follow an ideal free distribution pattern (see Fretwell and Lucas 1970; Fretwell 1972). Under such models, a dispersive fish species will occupy a pool depending on its quality, which is a function of intrinsic environmental characteristics and fish numbers in the pool (see Fraser and Sise 1980). In other words, pools with good environmental conditions will be occupied until high fish density is high enough to force some fish to move to other pools with poorer environmental conditions. The process may be repeated and lower quality pools may be become occupied by fewer fish along the stream. Unfortunately, no studies on summer steelhead have focused on this issue and those based on raibow trout seem to be contradictory. Campbell and Neuner (1985) noted a hierarchical relationship in rainbow trout, where the largest fish occupied the head of the pool, but Matthews et al. (1994) noted that thermally stratified poois rainbow trout did not interact for space in the 57 cooler areas of pools. In my study, fish abundance was relastvely low compared with past years (1. Roelof unpublished data). Thus I assumed that most of the pools was under their carrying capacity. However, such effects cannot be disregarded if run size increased. ['ISI V. MANAGEMENT CONSIDERATIONS AND FUTURE DIRECTIONS In the last decade a progressive depletion of salmonid stocks through the Pacific Northwest has occurred as a result of several factors (Bisson et al. 1992). In the North Umpqua River, the number of adult summer steelhead has declined in recent years (Fig. 11). a) _o L_. C C C I I I 1969 1971 1970 1972 I I 1973 1975 1974 I 1978 1977 1980 1979 1982 1981 1984 1983 1986 1985 1988 1987 1990 1989 1992 1991 Year Figure 11: Number of adult summer steelhead at Winchester Dam (North Umpqua River, OR), counted between May and November (1969-1992)(Oregon Fish and Wildlife Department, unpublished data). 59 Escapement of steelhead populations have decreased along the Oregon coast during this time (Everest et al. 1984; Konkel and McIntyre 1987). I believe that in order to improve that current status of summer steelhead in the Steamboat Creek basin, not only cool deep pools need to be managed and preserved, but also a broader perspective is required. I believe that the following management considerations are needed to improve or maintain pool conditions in Steamboat Creek. 5.1. Deep pools protection Efforts should be developed to protect deep cool pools that act as thermal refugia, even if they are marginal habitats. Loss of thermal refugia could have deleterious impact on summer steelhead populations. Coutant (1985; 1987) provided examples of how fish confined to small thermal refugia during the summer became stressed, had increased mortality and decreased reproductive potential. This has important consequences for salmonids which appear to have evolved through the selective pressure of bioenergetic efficiency (Brett 1971). Minimizing energetic costs has been recognized as an important goal of salmonids (Bernatchez and Dodson 1987). Fish must divert energy from growth and reproduction into survival strategies in stressful environments (Berman and Quinn 1992; Hall et al. 1992). Areas with water temperature above the optimum will increase metabolic costs (Dickson and Kramer 1971; Brett and Glass 1973; Smith and Li 1982), which may reduce energy available for reproduction (Gilhousen 1980). These potential constraints may be found during the summer in the Steamboat Creek basin. On the other hand, heavy use of pools by people should be of major concern. Some deep and critical pools for adult summer steelhead in Steamboat Creek are heavily used for swimming or diving. The potential impact of such activities must be investigated. In particular, I recommend a ban on any activity on pool 23 (see Fig.4). This pool is located immediately below the fish ladder at Steamboat Falls and may be used as a resting pools before fish ascend the ladder. 5.2. Considering water temperature as a source of pool complexity Faush and Bramblett (1991) stated that complex pools should be deeper than 1 m, have coarse substrate, vegetation cover or undercuts banks. Moore and Gregory (1989) found that pool complexity was determined mainly by boulders and woody debris. Other studies advocated hydraulic retention (Parsons et al. 1992), as source of habitat complexity or mixed structural variables with flow (Theurer et al. 1985). Because water temperature is a not a structural factor, its contribution to system complexity has often been neglected or not sufficiently considered in past studies. Previous research has described and measured proportion of habitat types, quantity of large woody debris, constrained and unconstrained reaches, habitat proportions and different geomorphological characteristics. Whereas these factors 61 affect fish abundance and distribution (e.g. Bisson et al. 1987), water temperature may be the most influential component during critical winter and summer periods (Adams et al. 1990). In the case of pools, seeps and thermal stratification offer a wider spectrum of microhabitat choices providing thermal complexity. However, maintenance of cool suitable temperature in reaches or habitat units cannot be isolated from watershed management (Heller et al 1983). Fish and land managers should work together to avoid erosion processes and temperature increases by protecting upper watersheds and riparian areas from logging practices and grazing effects. 5.3. Model testing I suggest caution be exercised in using results of the discrimiriant model in of pool use by adult summer steelhead in Steamboat Creek mother areas. Different authors noted that empirical models have very limited success when applied outside the geographical area where they were developed. For example, Lahyer et al. (1987) found that discriminant analysis failed in some cases to predict presence or absence of spotted bass (Micropterus punctatus) when applied outside the original region where the model was developed. These constraints are not only restricted to the use of multivariate analysis in stream ecology. Other approaches such as the habitat suitability curves, may also present problems. Kinzie and Ford (1988) concluded that habitat utilization curves cannot be transferred among streams. Morhardt (1987) reviewed the success of empirical models application for aquatic resources. My re-analysis of his data shows that only 18 % of the tested models agree with the expected results. This agrees with the poor success of extrapolated models in fisheries found by Fausch et al. (1989). Managers should be aware of the limited applicability of empirical models to regions outside the original areas. As stated by Box (1979) all models are wrong but some are more useful' and this principle fit into ecological studies. Finding the best model to predict pool use may imply a basin-by-basin study which is a costly alternative, but will yield more useful results. 5.4. Conduct studies on deep pool thermal dynamic Past results suggest that thermal characteristics of pools are still not well understood. Keller and Hofstra (1983), Bowlby and Roff (1984) and Matthews et al. (1994) identified cold groundwater and morphology as the main source of cold pool formation. Ozaki (1988) stated that stratification appeared to depend on hydrological and geomorphic characteristics. In turn, Neel (1951) found evidence that stratification could also be explained by the inlet temperature in the pool. My study in Steamboat Creek demonstrated that morphometry is a poor predictor of thermal stratification. I propose that more effort must be made to understand factors that determine thermal stratification in pools. Interesting progresses has been recently made by Moses (1984). Managers can apply this information to minimize human impact or to improve habitat conditions. For example, increasing riparian vegetation may 63 promote stratification during the day and provide colder habitats for trout. Cold water from springs can be diverted to pools to improve thermal conditions. In addition, some pools with good thermal conditions but poor cover, can be improved by increasing the depth via impounding the tail of the pool. Such procedure will in turn affect the water turnover ratio. This factor, in addition to width and depth has been identfied as a key parameter to promote thermal stratification. 5.5. Evaluate the stream thermoregulatory capacity Existence of cool spots in Steamboat Creek could be considered a limiting factor for adult summer steelhead. Thus, viewing thermal refuges in a spatialtemporal context could yield a more appropriate evaluation of how species can exploit their resources (Tracy and Christian 1986). Distribution of thermal refugia and reaches with unfavorable water temperature reaches would provide a valuable picture of a stream's thermoregulatory capability. Ultimately this information can be combined with other features such as cover, flow, depth and gradient on a geographic information system (GIS) platform to orientate management alternatives and evaluate the length of the stream offering suitable thermal and cover conditions. To accomplish this goal, managers need to be aware of routine analysis procedures (see Bartolow 1989) and check for appropriate sampling designs to achieve reliable information. Smith and Lavis (1975) pointed out that during the summer period marked differences can exist in water temperature along a stream course because of local topography and geological factors. Thermal changes are also not uncommon in very short distances (Bjornn et al. 1968). A difficulty often ignored in the literature is that standard statistical analysis might be inappropriate if spatial dependence is suspected for temperature processes. 5.6. Study of fish movements Questions dealing with pattern of pools occupancy when adults arrive in the basin, pooi carrying capacity and how possible past experience of individuals affect the use of pools, should receive further attention. Repeat spawners are not uncommon in summer steelhead stocks (Freese 1982). The percentage of repeat spawner varies among rivers systems (Roelof 1983). Since first spawners may have a different time of return than repeat spawners (Leider 1985), it would be interesting to see if earlier migrants select pools in a different way than do later migrants. It is still unknown if fish remain in the same pools over the summer. Dunn (1981) reported high pool fidelity for adults surveyed in Wooley Creek, California, but movement during summer storms were not uncommon (Freese 1982). It was interesting to note that some cool pools in Steamboat Creek were used considerably more than others. They probably had better conditions and high suitability. If fish follow an ideal free distribution, in years of large runs, managers should be prepared to protect optimal and marginal cool pools against poaching and other human disturbances. This protection should encompass the entire summer period. Tagging fish would be allow managers and researchers to gain an understanding of fish movements. Biotelemetry has detected fish movements and seasonal shifts in habitat used by chinook (Gray and Haynes 1979; Milligan et al. 1985) and Atlantic salmon (Atlantic salmon) (Webb 1992). Furthermore, the technique allows one to relate movement patterns and thermal habitats of salmonids (Haynes and Gerber 1989; Berman and Quinn 1991). Valuable information on life history patterns of steelhead has been collected by Everest (1973) in the Rogue River and Schroeder and Smith (1989) in the Deshutes River working on steelhead and rainbow trout, respectively. Tagged adult fish also allowed Freese (1982) to relate adult residence in pools with storms events in the North Fork Trinity River. In Steamboat Creek, past tagging experiments performed by Wroble (1990) have yield important information and should be continued. Furthermore, this study identified that pools use by adult summer steelhead appears to be a dynamic process, but followed different patterns depending of stream temperature. Since fish enter the basin from July to December it is virtually impossible to distinguish early and late migrants without marking techniques. Roelof (1983) recommended tagging experiments to answer similar questions in California. 5.7. Considering the riverscape perspective in deep pool use evaluation I suggest that a perspective that encompasses the riparian and aquatic river rT systems together is needed to understand why adult steethead are found in certain stream segments, and use or avoid certain deep pools within these streams. This approach can be termed the riverscape perspective. This approach considers the absolute and relative positions of each pool within a reach, a segment, a stream, and even the whole basin, and is not solely based on pool characteristics. Spatial (geographical) location implies not only inter-pools distances as an obvious parameter, but also inter-pools segments with their geomorphic characteristics. A traditional approach in stream fish ecology has been to consider fish abundance patterns based on habitat quality conditions while ignoring the potential effect of adjacent or surrounding habitats. In other words, studies on 'focal factors' predict the habitat use in time and space, but fail to consider the effect of habitat connectivity. This effect could be important in analyzing habitat use or preference by migratory fish. A riverscape approach takes this into account. Parsons et at. (1982) and Lanka et at. (1987) point out that trout habitat quality is related to the geomorphology of the basin; Freese (1982) reports that the distance to the closest downstream pool accounts for steelhead distribution in the New River, California. I believe that deep cool pools in Steamboat Creek are the most used habitats. Deep cool pools influence the distribution pattern of adult summer steelhead in the stream. Deep cool pools are embedded in a longitudinal matrix of unfavorable habitats, so that they appear as patches of suitable water temperature and depth. 67 In desert streams, pool patches were found to play a key role regulating fish survival (Deacon Williams and Williams 1989; Mundhal 1990). Fish movements have also been associated with patchy environments in large rivers (Welcomme 1985). Unfortunately, this approach has not been fully explored and applied by fisheries managers to explain fish distribution and migratory movements in small and moderate order streams (but see Fraser and Sise 1980). A riverscape framework can be useful to evaluate and predict fish movements and relate stream ecological processes within a landscape-watershed perspective (Shlosser 1991). This broad river perspective would be useful to analyze patterns of fish movements in relation to water temperature. Salmonids respond to natural temperature patterns in streams by moving upstream and downstream when temperature becomes unsuitable (Bjornn and Reisner 1992). Lack of riparian vegetation generates warm corridors that act as barriers between different stream segments, decreasing the connectivity among them. Because corridors exhibit a high edge/area ratio (Hobbs 1992), narrow streams like Steamboat or Canton Creek may be strongly affected by edge features such as shading. During the summer, development of such corridors are quasi instantaneous since direct insolation affects water temperature in a short time (Brown 1969; Rishel et al. 1982). Wroble and Roelof (1985) noted that some warm reaches with extensive bedrock exposed directly to sunlight are major reasons for increased water temperatures in Steamboat Creek. In the John Day basin, Oregon, Adams et al. (1990) found that the general passage of fish up and down a stream may be limited by reduced flows. In Steamboat Creek, exposed reaches lacking vegetation cover and with low flow fit the definition of warm corridors. Steamboat Creek can be interpreted as fragmented pattern of suitable habitat. The more fragmented a stream by warm segments, the less connectivity there is among suitable pools. In this sense, water temperature defines different subsystems in the stream; some pools act as cool patches, glides and riffles can be identified as warm corridors. Movement patterns within these subsystems may vary, and fish abundance may depend of environmental conditions in adjacent segments. In other words, fish may perceive deep pool features in the stream at different scales. At a minor scale, ecological attributes found within the pool, such as cover, substrate, flow and temperature (microhabitat features), define its quality. The fish response is to use or not use the pool. At a larger scale, pool selection can be governed by a comparative perspective among pool characteristics. Fish may be able to choose between pools, occupying the most suitable one. Finally, dispersion ability and inter-pool corridors characteristics will define pool connectivity, and therefore affect movement among pools. In summary, managers should realize that a hierarchical framework at a broad scale is required to enhance steelhead stock conditions in Steamboat Creek and other basins with identified water temperature problems. BIBLIOGRAPHY Adams, R.M., P.C. Kingeman and H.W. Li. 1990. A bioeeconomic analysis of water allocations and fish habitat enhancements, John Day basin, Oregon. Oregon State University, U.S. Geological Survey Grant Number 14-68-000G1479, 168 p. Anderson, J.W. and L. Miyajima. 1975. Analysis of the Vincent Creek fish rearing pool project. U.S. Department of the Interior-Bureau of Land Management, Technical Note 274, 25 p. Baldes, R.J. and R.E. 1969. Physical parameters of microhabitats occupied by brown trout in a experimental flume. Transactions of the American Fisheries Society 98: 230-238. Baltz, D.M., B. Vondracek, L.R. Brown and P.B. Moyle. 1987. Influence of temperature on microhabitat choice by fishes in a California stream. Transactions of the American Fisheries Society 116: 12-20. Baltz, D.M., B. Vondracek, L.R. Brown and P.B. Moyle. 1991. Seasonal changes in microhabitat selection by rainbow trout in a small stream. Transactions of the American Fisheries Society 120: 166-176. Barnhart, R. A. 1986. Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (Pacific Southwest)--steelhead. U.S. Fish WildI. Serv. Biol. Rep. 82 (11.60). U. S. Army Corps of Engineers, TR EL-824, 21 p. Bartholow, J.M. 1989. Stream temperature investigations: Field and analytical methods. Iristream Flow Information Paper No 13. United States of Fish and Wildlife Services Biological Report 89 (17), 139 p. Bartholow, J.M. and W. Slauson. 1990. Questions on habitat preference. North American Journal of Fisheries Management 10: 362-363. Beauchamp, J.J, S.W. Christensen and E.P. Smith. 1992. Selection of factors affecting the presence of brook trout (Salvelinus fontinalis). Canadian Journal of Fisheries and Aquatic Sciences 49: 597-608. Bell, M.C. 1986. Fisheries handbook of engineering requirements and biological criteria. U.S. Army Corps of Engineers, Office of the Chief of Engineers, Fish Passage Development and Evaluation Program, Portland, Oregon, 290 p. 70 Berman, C.H. and T.P. Quinn. 1991. Behavioral thermoregulation and homing by spring chinook salmon, Oncorhynchus tshawytscha (Walbaum), in the Yaquima river. Journal of Fish Biology 39: 301 -31 2. Bernatchez, L. and J.J. Dodson. 1987. Relationship between bloenergetics and behavior in anadromous fish migrations. Canadian Journal of Fisheries and Aquatic Sciences 44: 399-497. Beschta, R. L. and W. S. Platts. 1986. Morphological features of small streams: significance and function. Water Research Bulletin 22: 369-379. Beschta, R.L, R.E. Bilby, G.W. Brown, L.B. Hoitby and T.D. Hofstra. 1987. Stream temperature and aquatic habitat: fisheries and forestry interactions. Pages 191-232 in Salo E.O. and T.W. Cundy (eds.) Streamside management: forestry and fishery interactions. Institute of Forest Resources, University of Washington, Contribution No 57. Bibby, C., N.D. Burgess and D.A. Hill. 1992. Bird census techniques. Academic Press, London, 257 p. Bilby, R.E. and L.J. Wasserman. 1989. Forest practices and riparian management in Washington state: Data based regulation development. Pages 87-94 in Gresswell R., B.A. Barton and J. L. Kershner (eds.) Practical approaches to riparian resource management. An educational workshop. U.S. Bureau of Land Management, Billings, Montana, 193 p. Binns, N. A. 1982. Habitat quality index procedures manual. Wyoming Game and Fish Department, 209 p. Binns, N. A and F. M. Eiserman. 1979. Quantification of fluvial trout habitat in Wyoming. Transactions of the American Fisheries Society 108: 21 5-228. Bisson, P.A., J.L. Nielsen, R.A. Plamason and L.E. Grove. 1982. A system of naming habitat types in small streams, with examples of habitat utilization by salmonids during low streamfiow. Pages 62-73 in N. Armantrout (ed.) Acquisition and utilization of aquatic habitat inventory information. American Fisheries Society, 375 p. Bisson, P.A., R.E. Bilby, M.D. Bryant, C.A.. Dollof, G.B. Grette, R.A. House, M.L. Murphy, K.V. Koski and J.R. Sedell. 1987. Large woody debris in forested streams in the Pacific Northwest: Past, present and future. Pages 143-190 in Salo E.O. and T.W. Cundy (eds.) Streamside management: forestry and 71 fishery interactions. Institute of Forest Resources, University of Washington, Contribution No 57. Bisson, P., T. A. Quinn, G. H. Reeves and S. V. Gregory. 1992. Best management practices, cumulative effects and long-term trends in fish abundance in Pacific Northwest river systems. Pages 189-232 in R. J. Naiman (ed.) Watershed management: balancing sustainability and environmental change. Springer-Verlag, New York, 542 p. Bjornn, T.C., D.R. Craddock and D.R. Corley. 1968. Migration and survival of Redfish lake, Idaho, sockeye salmon, Oncorhynchus keta. Transactions of American Fisheries Society 97: 360-373. Bjornn, T.C. and D.W. Reiser. 1992. Habitat requirements of salmonids in streams. Pages 83-138 in W. A. Meehan (ed.)Influences of forest and rangeland management on salmonids fishes and their habitats. American Fisheries Society Special Publication 19, Bethesda, Maryland. Boussu, M.F. 1954. Relationship between trout populations and cover on a small stream. Journal of Wildlife Management 18: 229-239. Bovee, K. D. 1978. Probability of use criteria for the familia Salmonidae. U.S. Fish and Wildlife Service Biological Services Program FWS/OBS-78/07. Bovee, K. and 1. Cochnauer. 1977. Development and evaluation of weighted criteria, probability-of-use- curves for instream flow assessment: fisheries. U.S. Fish and Wildlife Services Biological Services Program FWS/OBS77/63. Bovee, K. and J.R. Zuboy. 1986. Proceedings of a workshop on the development and evaluation of habitat suitability criteria. U.S. Fish and Wildlife Services Biological Report 88(11), 407 p. Bowlby, J.N and J.G. Imhof. 1989. Alternative approaches in predicting trout populations from habitat in streams. Pages 318-332 in Gore J.A. and G.E. Petts (eds.) Alternatives in regulated river management CRC Press Inc., Boca Raton, Florida. Bowiby, J.N. and J.C. Roff. 1986. Trout biomass and habitat relationship in southern Ontario streams. Transactions of the American Fisheries Society 115: 503-513. Box, C.E. 1979. Robustness in the strategy of scientific model building. Pages 201- 236 in Launer R.L.and G.N. Wilkinson (eds.) Robustness in statistics, 72 Academic Press, New York, 296 p. Brett, J.R. 1971. Energetic response of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of the sockeye salmon (Oncorhynchus nerka). American Zoologist 11: 99-113. Brett, J.R. and N.R. Glass. 1973. Metabolic rates and critical swimming speeds of sockeye salmon (Oncorhynchus nerka) in relation to size and temperature. Journal of Fisheries Research Board of Canada 30: 379-387. Brown, G.W. 1969. Predicting temperatures of small streams. Water Resources Research 5: 68-75. Brown, G. W. 1971. Water temperature in small streams as influenced by environmental factors and logging. Pages 175-181 in Krygier, J.T. and J.D.Hall (eds.) Proceedings of a symposium forest land uses and stream environment, Oregon State University, 252 p. Brown, G.W. and J.T. Krygier. 1967. Changing water temperatures in small mountain streams. Journal of Soil and Water Conservation 22: 242-244. Brown, G.W. and J.T. Krygier. 1970. Effect of clearcutting on stream temperature. Water Resources Research 6: 1133-1139. Brown, G., G.W. Swank and J. Rothacher. 1971. Water temperature in the Steamboat drainage. Pacific Northwest Forest and Range Experimental Station, Portland Oregon, 17 p. Buttler, R.L. and V.M. Hawthorne. 1968. The reactions of dominant trout to changes in overhead artificial cover. Transactions of the American Fisheries Society 97: 37-41. Campbell, R.F. and J.H. Neuner. 1985. Seasonal and diurnal shifts in habitat utilized by resident rainbow trout in Western Washington Cascade mountain streams. Pages 39-48 in Olsen F.W., R.G. White and R.H. Hamre (eds.) Proceedings of the symposium on small hydropower and fisheries. American Fisheries Society, Western Division, Bethesda, Maryland. Capone, T.A. and J.A. Kushlan. 1991. Fish community structure in dry-season stream pools. Ecology 72: 983-992. Carpenter, S.R. 1983. Lake geometry: implications for production and sediment accretion rates. Journal of Theoretical Biology 105: 273-286. 73 Cherry, D.S., K L. Dickson and J. Cairns Jr. 1975. Temperature selected and avoided by fish at various acclimation temperature. Journal of Fisheries Research Board of Canada 32: 485-491. Cherry, D.S., K.L. Dickson, J.Cairns Jr. and J. Stauffer. 1977. Preferred, avoided and lethal temperatures of fish during rising temperature conditions. Journal of Fisheries Research Board of Canada 34: 239-246. Clapp, D.F. and R.D. Clark Jr. 1990. Range, activity and habitat of large, free-ranging brown trout in a Michigan stream. Transactions of the American Fisheries Society 119:1022-1034. Cochran, W. G. 1964. On the performance of the linear discriminant function. Technometrics 6: 179-189. Cohen, J. 1960. A coefficient of agreement for nominal scales. Education and Psychological Measurements 20: 37-46. Cohen, J. 1988. Statistical power analysis for the behavioral sciences, Lawrence Erlbaum Assoc. PubI., Hillsdale, 566 p. Collins, C. S. 1971. North Umpqua case study - an introduction. Pages 225-226 in Krygier, J.T. and J.D.Hall (eds.) Proceedings of a symposium forest land uses and stream environment, Oregon State University, 252 p. Coutant, C.C. 1985. Striped bass, temperature and dissolved oxygen: A speculative hypothesis for environmental risk. Transactions of the American Fisheries Society 114: 31-61. Coutant, C.C. 1987. Thermal preferences: when does an asset become a liability. Environmental Biology of Fishes 18: 161-172. Dambacher, J. M. 1991. Distribution, abundance and emigration ofjuvenile steelhead (Oncorhynchus mykiss) and analysis of stream habitats in the Steamboat Creek basin, Oregon. M.S. Thesis, Oregon State University, 129 p. De Vore, P.W. and R.J. White. 1978. Daytime responses of brown trout (Salmo trutta) to cover stimuli in stream channels. Transactions of the American Fisheries Society 107: 763-771. Deacon Williams, C. and J.E. Williams. 1989. Refuge management for the threatened Railroad Valley springfish in Nevada. North American Journal of Fisheries Management 9: 465-470. 74 Dickson, LW. and R.H. Kramer. 1973. Factors influencing scope for activity and active and standard metabollsm of rainbow trout (Salmo gairdneri). Journal of Fisheries Research Board of Canada 28: 587-596. Dolloff, A.C. and G.H. Reeves. 1990. Microhabitat partitioning among streamdwelling juvenile coho salmon, Oncorhynchus kisutch and Dolly Varden, Salvelinus malma. Canadian Journal of Fisheries and Aquatic Sciences 47: 2297-2306. Duff, 0. A. and J. L. Cooper. 1976. Techniques for conducting stream habitat survey on national resource land. T/N 283, U.S. Department of the Interior, Bureau of Inland Management, 72 p. Dunn, P.L. 1981. Adult summer steelhead in Wooley Creek, California: migration behavior and holding habitat. M.S. Thesis, Humboldt State University, Arcata, 83 p. Elser, A.A. 1968. Fish populations of a trout stream in relation to major habitat zones and channel alterations. Transactions of the American Fisheries Society 97: 389-397. Everest, F.H. 1973. Ecology and management of summer steelhead in the Rogue River. Fisheries Research Report 7, Oregon State Game Commission, 48 p. Everest, Ff1. and D.W. Chapman. 1971. Habitat selection and spatial interaction by juvenile chinook salmon and steelhead trout in two Idaho streams. Journal of Fisheries Research Board of Canada 29: 91-100. Everest, F.l-L, J.R. Sedell, G.H. Reeves and J. Wolfe. 1984. Fisheries enhancement in the Fish Creek basin--an evaluation of in-channel and off- channel projects, 1984. U. S. Department of Energy Boneville Power Administration Division of Fish and Wildlife, Annual Report, 228 p. Fausch, K.D. and R.G. Bramblett. 1991. Disturbance and fish communities in intermittent tributaries of a western great plains river. Copeia 1991: 659-674. Faush, K.D. and T.G. Northcote. 1992. Large woody debris and salmonid habitat in a small coastal British Columbia stream. Canadian Journal of Fisheries and Aquatic Sciences 49: 682-693. Fausch, K.D., C.L. Hawkes, M.G. Parsons 1989. Models that predict standing crop of stream fish from habitat variables:1950-1985. USDA Forest Services, Portland, Oregon, Pacific Northwest Research Station. 75 Fleiss, J.L. 1977. Statistical methods for rates and proportions, John Wiley and Sons, New York, 321 p. Fontaine, B. 1987. An evaluation of the effectiveness of instream structure for steelhead trout rearing habitat in the Steamboat Creek basin. M.S. Thesis, Oregon State University, 68 p. Fraley, J.J. and P.J. Graham. 1982. Physical habitat, geologic bedrock types and trout densities in tributaries of the Flathead river drainage, Montana. Pages 178-190 in Armantrout N. (ed.) Acquisition and utilization of aquatic habitat inventory information. American Fisheries Society, 375 p. Fraser, D. F and 1. E. Sise. 1980. Observations on stream minnows in a patchy environment: a test of a theory of habitat distribution. Ecology 61: 790-797. Freese, J.L. 1982. Selected aspects of the ecology of adult summer steelhead in Trinity River, California. M.S. Thesis, Humboldt State University, Arcata, CA, 96 p. Fretwell, S.D. 1972. Population in a seasonal environment. Princeton University Press, New Jersey, 217 p. Fretwell, S. D. and H. L. Lucas, Jr. 1970. On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheoretica XIX: 16-36. Garside, E.T. and J.S. Tait. 1958. Preferred temperature of rainbow trout (Salmo gairdneri) and its unusual relationship to acclimation temperature. Canadian Journal of Zoology 36: 536-567. Gibson, R.J. and M.H. Keenleyside. 1966. Responses to light of young Atlantic salmon (Salmo salar) and brook trout (Salvellnus fontinalis). Journal of Fisheries Research Board of Canada 32: 1007-1024. Gilhousen, P. 1980. Energy sources and expenditures in Fraser River sockeye salmon during their spawning migration. International Pacific Salmon Fisheries Commission Bulletin X)(ll, 48 pp. Gorman O.T. and J.R. Karr. 1978. Habitat structure and stream fish communities. Ecology 59: 507-515. Gray, J.C. and J.M. Edington. 1969. Effect of woodland clearance on stream temperature. Journal of Fisheries Research Board of Canada 26: 399-403. Green, R.H. 1979. Sampling design and statistical methods for environmental biologists, John Wiley and Sons, New York, 257 p. Grette, G. B. 1985. The role of large organic debris in juvenile salmonid rearing habitat in small streams. M.S. Thesis, University of Washington, 105 p. Grossman, GD. and M.C. Freeman. 1987. Microhabitat use in a stream fish assemblage. Journal of Zoology of London 212: 151-176. Hair, J. F., Jr., P. E. Anderson and R. L. Tatham. 1987. Multivariate data analysis, Mcmillan Pubi. Co., New York, 449 p. Hall, C.A., J.A. Standford and F.R. Hauer. 1992. The distribution and abundance of organisms as a consequence of energy balances along multiple environmental gradients. Oikos 65: 377-390. Hampton, M. and M. Aceituno. 1988. Direct observation techniques for habitat use criteria development ont the Trinity River, Trinity county, California. Pages 159-180 in Bovee K. and J.R. Zuboy (eds.) Proceedings of a workshop on the development and evaluation of habitat suitability criteria. U.S. Fish and Wildlife Services Biological Report 88(11), 407 p. Harshbarger, T. J. and H. Bhattacharya. 1981. An application of factor analysis in an aquatic habitat study. Pages 180-184 in Capen D.E. (ed.) The use of multivariate statistics in studies of wildlife habitat, USDA Forest Services General Technical Report, RM-87, 231 p. Haynes, J.M. and G.P. Gerber. 1989. Movements and temperatures of radiotagged salmonines in lake Ontario and comparisons with other large aquatic systems. Journal of Freshwater Ecology 5: 197-204. Heggens, J.A. 1988. Physical habitat selection by brown trout (Salmo trutta) in riverine systems. Nordic Journal of Freshwater Research 64: 74-90 Hokkanson, K.E. 1977. Temperature requirements od some percids and adaptations to the seasonal temperature cycle. Journal of Fisheries Research Board of Canada 34: 1524-1550. Heggens, J.A. 1988. Effects of short-term flow fluctuations on displacement of, and habitat use by, brown trout in a small stream. Transactions of the American Fisheries Society 117: 336-344. Helfman, G.S. 1981. The advantage of fishes of hovering in shade. Copela 1981: 392-400. Heller, D.A., J.R. Maxwell and M. Parsons. 1983. Modeling the effects of forest management on salmonid habitat. Forest Services, Pacific Northwest region, Siuslaw National Forest, 63 p. Helm, W. T. ed. 1985. Aquatic habitat inventory: glossary and standards methods. American Fisheries Society, Western Division, Bethesda, Maryland, 35 p. Higgins, P. and W.M. Kier. 1992. A plan for restoring biodiversity of anadromous fish resources of the Klamath River. Pages 217-225 in R. Harris and D. Erman (eds.) Proceeding of the symposium of biodiversity of Northwestern California, October 28-30, 1991, Santa Rosa, California, University of California, Berkeley. Hinch, S.G., N.C. Collins and H.H. Harvey. 1991. Relative abundance of littoral zone fishes: biotic interactions, abiotic factors and postglacial colonization. Ecology 72: 314-1324. Hobbs, R. J. 1992. The role of corridors in conservation: solution or bandwagon. Trends in Ecology and Evolution 7: 389-391. Holaday, S.A. 1992. Summertime water temperature trends in Steamboat Creek basin, Umpqua National Forest. M.S. thesis, Oregon State University, 128 p. Hoitby, L.B. 1988. Effect of logging on stream temperature in Carnation Creek, British Columbia and associated impacts on the coho salmon (Oncorhynchus kisutch). Canadian Journal of Fisheries and Aquatic Sciences 45: 502-515. Hostetler, S.W. 1991. Analysis and modeling of long-term stream temperatures on the Steamboat Creek basin, Oregon: implications for land use and fish habitat. Water Research Bulletin 27: 637-647. Hubbard, L.E., T. Herrett, R. Kraus and C. Kroll. 1990. Water resources data for Oregon, water year 1990. Vol 2. Western Oregon. USGS/WRD/HD-91/250. Irvine, J.R., 1G. Jowett and D. Scott. 1987. A test of the instream flow incremental methodology for underyearling rainbow trout, Salmo gairdneri in experimental New Zealand streams. New Zealand Journal of Marine and Freshwater Research 21: 35-90. Jones, W.E. 1979. Summer steelhead (Sa/mo gairdneri) in the Middle Fork Eel River and their relationship to environmental changes, 1966 through 1978. California Department of Fish and Game, Anadromous Fisheries Branch Administration, Report 80-2, 25 p. Jones, WE. 1982. Summer steelhead survey Middle Fork Eel River Trinity and Mendocino counties. Region 3, California Department of Fish and Game, November 1992 (mimeo). Jones, W.E. 1992. Historical distribution and recent trends of summer steelhead, Oncorhynchus mykiss, in the Eel River, California. Region 3, California Department of Fish and Game, January 1992 (mimeo). Jowett, I.G. 1990. Factors related to the distribution and abundance of brown trout and rainbow trout in New Zealand clear-water rivers. New Zealand Journal of Marine and Freshwater Research 24: 429-440. Kahn, H. and C.T. Sempos. 1989. Statistical methods in epidemiology. Oxford University Press, New York, 293 p. Kaya, C.M., L.R. Kaeding and D.E. Burkhalter. 1977. Use of cold-water refuge by rainbow trout and brown trout in a geothermally heated stream. Progressive Fish Culturist 39: 37-39. Keller, E.A. and T.D. Hofstra. 1983. Summer cold pools in Redwood Creek near Orick, California and their importance as habitat for anadromous salmonids. Pages 221-224 in Van Riper C. Ill, L. Whiting and M. Murphy (eds.) Proceedings of the First Biennial Conference of Research in California's National Parks. University of California, Davies, 310 p. Kendall, M. 1980. Multivariate analysis. McMillan Pubi. Co., Inc., New York, 210 p. Kendall, M.G. and A. Stuart. 1966. The advanced theory of statistics, vol Ill. Griffin ed., London, 552 p. Klecka, W.R. 1980. Discriminant analysis. Sage University paper series. Quantitative Applications in Social Sciences, series no. 07-019, Beverly Hills, London, 71 p. Konkel, G.W. and J.D. McIntyre. 1987. Trends in spawning populations of Pacific anadromous salmonids. Fish and Wildlife Technical Report 9, 25 p. Lanka, ftP., W.A. Hubert and T.A. Wesche. 1987. Relations of geomorphology to stream habitat and trout standing stock in small Rocky mountain streams. Transactions of the American Fisheries Society 116: 21-28. Lantz, R. L. 1970. Influence of water temperature on fish survival, growth and 79 behavior. Pages 182-193 J. Morris (ed.) Proceedings of symposium on forest land uses and the stream environment. Oregon State University, in Corvallis. Larscheid, J.G. and W.A. Hubert. 1992. Factors influencing the size structure of brook trout and brown trout in southeastern Wyoming mountain streams. North American Journal of Fisheries Management 12: 109-117. Layher, W.G. and O.E. Maughan. 1987. Spotted bass habitat suitability related to fish occurrence and biomass and measurements of physicochemical variables. North American Journal of Fisheries Management 7: 238-251. Legendre, L. and P. Legendre. 1983. Numerical ecology. Elsevier Scientific Publication Company, Amsterdam, 419 p. Leider, S.A. 1985. Precise timing of upstream migrations by repeat steelhead spawners. Transactions of the American Fisheries Society 114: 906-908. Lewis, S. 1969. Physical factors influencing fish populations in pools of a trout stream. Transactions of the American Fisheries Society 98: 14-19. Marlega, R.R. 1971. Streamside strips and management problems. Pages 228-229 in Krygier, J.T. and J.D. Hall (eds.) Proceedings of a symposium forest land uses and stream environment, Oregon State University, 252 p. Matthews, W.J. 1990. Spatial and temporal variation in fishes of riffle habitats: a comparison of analytical approaches for the Roanoke River. American Midland Naturalist 124: 31-45. Matthews, K.R., N.H. Berg and D.L. Azuma and 1. R. Lambert 1994. Cool water formation and trout habitat use in a deep pool in the Sierra Nevada, California. Transactions of the American Fisheries Society 123: 549-564. McCabe, G.P. Jr. 1975. Computations for variable selection in discriminant analysis. Technometrics 17: 103-109. McCauley, R.W. and N.W. Huggins. 1979. Ontogenetic and non-thermal seasonal effects on thermal preferenda of fish. American Zoologist 19: 267-271. McCauley, R. W. and W. L. Pont 1971. Temperature selection of rainbow trout fingerlings in vertical and horizontal gradients. Journal of Fisheries Research Board of Canada 28: 1801-1804. McMichael, G. and C.M. Kaya. 1991. Relations among stream temperature, angling success for rainbow trout and brown trout, and fisherman satisfaction. North American Journal of Fisheries Management 11: 190-199. Meisner, J.D. 1990. Potential loss of thermal habitat for brook trout, due to climatic warming in two southern Ontario streams. Transactions of the American Fisheries Society 119: 282-291. Mesick, C.F. 1988. Effects of food and cover on numbers of Apache and brown trout establishing residency in artificial stream channels. Transactions of the American Fisheries Society 117: 431-431. Milligan, P.A., W.O. Publee, D.D. Cornett and R.A. Johnston. 1985. The distribution and abundance of chinook salmon (Oncorhynchus in the upper Yukon river basin as determined by a radiotagging and spaghetti tagging program: 1982-1982. Canadian Technical tshawytscha) Report in Fisheries Aquatic Sciences 1352, 157 p. Modde, T., R.C. Ford and M.G. Parsons. 1991. Use of habitat-based stream classification system for categorizing trout biomass. North American Journal of Fisheries Management 11:305-311. Moore, K. M. and S. V. Gregory. 1989. Geomorphic and riparian influences on the distribution and abundance of salmonids in a cascade mountain stream. Pages 256-261 in Proceedings of the California riparian systems conference September 22-24, 1988. Protection, management and restoration for the 1990's. U.S. Department of Agriculture, Washington DC. Morhardt, J. E. 1986. Iristream flow methodologies. EA-4819, Research Project 2194-2, EAEngineering, Science and Technology, Inc., Lafayette, California. Morhardt, J.E. and D.F. Hanson. 1988. Habitat availability considerations in the development of suitable criteria. Pages 292-407 in Bovee K. and J. R. Zuboy. (eds.) Proceedings of workshop on the development and evaluation of habitat suitability criteria. U.S. Fish and Wildlife Services Biological Report 88(11), 407 p. Moring, J. R. 1975. The Alsea watershed study: Effects of logging on the aquatic resources on three headwater streams of the Alsea River, Oregon. Fishery Research Report 9, Oregon Department Fish & Wildlife, Corvallis, Oregon, 39 p. Morrison, D.F. 1967. Multivariate statistical methods. McGraw-Hill, New York. Morrison, D.F. 1969. On the interpretation of discriminant analysis. Journal of [.. Marketing Research 6:156-163. Moses, C.G. 1984. Pool morphology of Redwood Creek, California. M.S. Thesis, University of California, Santa Barbara, 117 p. Mundahl, N. D. 1990. Heat death of fish in shrinking stream pools. American Midland Naturalist 123: 40-46. Nakamoto, R. 1994. Characteristics of pools used by adult summer steelhead oversummering in the New River, California. Transaction of the American Fisheries Society 123: 757-765. Neel, J.K. 1951. Interrelations of certain physical and chemical features in a headwater limestone stream. Ecology 32: 368-391. Nelson, R.L., W.S. Platts, D.P. Larsen and S.E. Jensen. 1992. Trout distribution and habitat in relation to geology and geomorphology in the North Fork Humboldt river drainage, Northeastern Nevada. Transaction of the American Fisheries Society 121: 405-426. Nickelson, T.E., W.M. Beidler and M.J. Willis. 1979. Streamflow requirements of salmonids. Federal Aid Progress Report Fisheries, Oregon Department of Fish and Wildlife, AFS-62, 29 p. Nielsen, J.L. 1992. The role of cold-pool refugia in the freshwater fish assemblage in Northern California rivers. Pages 79-87 in Kerner, H.M (ed.) Proceeding of the symposium of biodiversity of Northwestern California, October 28-30, 1991, Santa Rosa, CA. University of California, Wildiand Resources Center Report No 29, 316 p. Nielsen, J.L, T.E Lisle and V. Ozaki. 1994. Thermally stratified pools and their use by steelhead in northern California stream. Transaction of the American Fisheries Society 123: 613-626. Northcote, T.G. 1992. Migratory and residency in stream salmonids- some ecological considerations and evolutionary consequences. Nordic Journal of Freshwater Research 67: 5-17. Olson-Rutz, K.M. and C.B. Marlow. 1992. Analysis and interpretation of stream channel cross-sectional data. North American Journal of Fisheries Management 12: 55-61. Oswood, M. E. and W. E. Barber. 1982. Assessment of fish habitat in streams: goals, constraints and a new technique. Fisheries 7: 8-11. Ozaki, V. L. 1988. Geomorphic and hydrologic conditions for cold pool formation on Redwood Creek, California. Reedwood National Park Research and Development Technical Report. Reedwood National Park, Arcata, CA. 57 p + appendices. Parsons, M., J.R. Maxwell and D. Helter. 1982. A predictive fish habitat index model using geomorphic parameters. Pages 85-91 in Armantrout N. (ed.) Acquisition and utilization of aquatic habitat inventory information. American Fisheries Society, 375 p. Parsons, T. N., H. W. Li and 0. A. Lamberti. 1992. Influence of habitat complexity on resistance to flooding and resilience of stream fish assemblages. Transactions of the American Fisheries Society 121: 427-436. Picard, R.R. and K.N. Berk. 1990. Data splitting. American Statistics 44: 140-147. Pimentel, R.A. 1979. Morphometrics, the multivariate analysis of biological data. Kendall/Hunt Publishing Company, Dubuque, Iowa, 276 p. Platts, W.S. 1979. Relationships among stream order, fish populations and aquatic geomorphology in an Idaho river drainage. Fisheries 4(2): 5-9. Platts, W.S., W.F. Megahan and G.W. Minshall, 1983. Methods for evaluating stream, riparian and biotic conditions. General Technical Report INT-138. Ogden, UT:U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range experimental Station, 70 p. Platts, W.S. et at. 1987. Methods for evaluating riparian habitats with applications to management. General Technical Report INT-221. Ogden, UT: U.S. Department of Agriculture, Forest Services, Intermountain Forest and Range experimental Station, 177 p. Rahel, F.J. 1984. Factors structuring fish assemblages along a big lake successional gradient. Ecology 65: 1276-1289. Raleigh, R. F., 1. Hickman, R. Solomon and P. Nelson. 1984. Habitat suitability information: Rainbow trout. Fish and Wildlife Services, FWS/OBS-82/10.60, 64 p. Reeves, G.H., G.H. Everest and J.D. Hall. 1987. Interactions between the redside shinner (Richardsonius baleatus) and the steelhead trout (Salmo gairdneri) on eastern Oregon. Canadian Journal of Fisheries and Aquatic Sciences 44: 1603-1613. Reeves, G.H., J.D. Hall, T.D. Roelof, T.L. Hickman and C.O. Baker. 1991. Rehabilitating and modifying stream habitats. Pages 519-556 in W.R. Meehan (ed.) Influence of forest and rangeland management on salmonid fishes and their habitats. American Fisheries Society, Special Publication 19. Reynolds, W.W. 1977. Temperature as a proximate factor in orientation behavior. Journal of Fisheries Research Board of Canada 34: 734-739. Reynolds, W.W. and M.E. Casterlin. 1979. Behavioral thermoregulation and the "final preferendum" paradigm. American Zoologist 19: 211-224. Rinne, J. N. 1982. Problems associated with habitat evaluation of an endangered fish in headwater environments. Pages 202-209 in N. B. Armantrout (ed.) Acquisition and utilization of aquatic habitat inventory information. American Fisheries Society Western Division, Bethesda, Maryland. Rinne, J. N. 1978. Development of methods of population estimation and habitat evaluation for management of the Arizona and Gila trouts. Pages 113-123 in J.R. Moring (ed.) Proceedings wild trout-catchable trout symposium, Oregon Department of Fisheries and Wildlife, Corvallis. Rishel, G.B., J.A. Lynch and E.S. Corbett. 1982. Seasonal stream temperature changes following forest harvesting. Journal of Environmental Quality 11: 112-116. Roelof, T.D. 1983. Current status of California summer steelhead (Salmo gairdneri) stocks and habitat and recommendations for their management. USDA Forest Services, Region 5, San Francisco, California, 77 p. Roelof, T.D., M.S. Hill and J.J. Wroble. 1983. Juvenile salmonid densities in eight north Umpqua River tributaries, summer 1983. Report presented to Steamboat Ranger District, Umpqua National Forest, Oregon Department Fish and Wildlife, l6p. SAS, 1985. SAS users's guide: statistics. SAS Institute, Gary, North Carolina, USA. Sedell, J.R., G.H. Reeves and F.H. Everest. 1986. Reoccupying our habitat losses with a riparian vegetation strategy. Pages 30-39 in D. Gutrie (ed.) Proceedings of wild trout, steelhead and salmon in the 21 at century, Oregon Sea Grant No. ORESU-W-86-002, Oregon State University. Scarnecchia, D.L. and E.P. Bergersen. 1987. Trout production and standing crop in Colorado's small streams, as related to environmental features. North American Journal of Fisheries Management 7: 315-330. Schiessinger, D.A. and H.A. Regier. 1983. Relationship between environmental temperature and yields of subartic and temperate zone fish species. Canadian Journal of Fisheries Research Board of Canada 34: 1784-1791. Schiosser, l.J. 1991. Stream fish ecology: a landscape perspective. Bioscience 41: 704-712. Seber, G.A. 1984. Multivariate observations. John Wiley & Sons, New York, 686 p. Sheppard, J.D. and J.H. Johnson. 1985. Probability-of-use for depth, velocity, and substrate by subyearling coho salmon and steelhead in lake Ontario tributary streams. North American Journal of Fisheries Management 5: 277282. Skeesick, D. 1989. Biology of the Bull trout, Salvelinus confluetitus. Willamette National Forest, Eugene Oregon, 53 p. Smith, J.J. and H.W. Li. 1973. Energetic factors foraging tactics of juvenile steelhead trout, Salmo gairdneri. Pages 173-180 in 0. L. Noakes (ed.) Predators and prey in fishes. Dr. W. Junk Publishers, The Hague, Netherlands. Smith, K.and M. E. Lavis. 1975. Environmental influences on the temperature of a small upland stream. Oikos 26: 228-236. Spigarelli, S.A., G.P. Romberg, W. Prepejchal and M.M. Thommes. 1974. Bodytemperature characteristics of fish at thermal discharge on lake Michigan. Pages 119-132 in J.W. Gibbons and R.R. Sharitz (eds.) Thermal ecology, U.S. Atomic Energy Commission, Oakridge. Stauffer, J.R. Jr. 1980. Influence of temperature on fish behavior. Pages 103-141 in C.H. Hocutt, J.R. Stauffer, J.E. Edinger, L.W. Hall Jr. and R.P. Morgan II (eds.) Power Plants. Effects on fish and shellfish behavior, Academic Press, New York, 346 p. Stevens, J. 1986. Applied multivariate statistics for the social sciences. Lawrence Erlbaum Assoc., Pubi., Hilisdale, New Jersey, 515 p. Stewart, P. A. 1970. Physical factors influencing trout density in a small stream PhD Thesis, Colorado State University, Fort Collins, Colorado. Tabachnick, B. G. and L. S. Fidell. 1989. Using rnu(tivariate statistics. Harper & Row, Pubi., New York, 746 P. Theurer, F. 0., I. Lines and T. Nelson. 1985. Interaction between riparian vegetation, water temperature and salmonid habitat in the Tucannon river. Water Research Bulletin 27: 53-64. Titus, K., J.A. Mosher and B.K. Williams. 1984. Chance-corrected classification for use in discriminant analysis: ecological applications. American Midland Naturalist 111: 1-11. Tonn, W.M., J.J. Magnuson and A. Forbes. 1983. Community analysis in fishery management: an application with northern Wisconsin lakes. Transaction of the American Fisheries Society 112: 368-377. Tracy, R. C. and K. A. Christian. 1986. Ecological relations among space, time and thermal niche axis. Ecology 67: 609-615. U.S. Environmental protection agency. 1986. Quality criteria for water 1986. EPA 440/5-86-001. Government Printing Office, Washington D.C. Waite, l.R. and R.A. Barnhart. 1992. Habitat criteria for rearing steelhead: a comparison of site-specific and standard curves for use in the instream flow methodology. North American Journal of Fisheries Management 12: 40-46. Van Home, B. 1983. Density as a misleading indicator of habitat quality. Journal of Wildlife Management 47: 893-901. Webb, J. 1992. The behavior of adult salmon (Salmon salar L.) in the River Tay as determined by radio telemetry. Scottish Fisheries Report 52, 18 p. Welcomme, A. L. 1985. River fisheries. FAO Technical Paper 262. Food and Agriculture Organization of the United Nations, Rome, Italy. Welch, P.S 1948. Limnological methods, Blakiston Co., Phadelphia, 538 p. Wesche, T., C. Goertler and C. Frye. 1985. Importance and evaluation of instream and riparian cover in smaller trout streams. Pages 325-328 in Johnson R. et al. (eds.) Riparlan ecosystems and their management: reconciling conflicting uses. USDA Forest Services General Technical Report RM-120, Fort Collins, Colorado, 523 p. Wesche, T., C. M. Goertler and W. A. Hubert. 1987 a. Modified habitat suitability index model for brown trout in southeastern Wyoming. North American Journal of Fisheries Management 7: 232-237. Williams, B. K. 1983. Some observations on the use of discriminant analysis in ecology. Ecology 68: 1283-1291. Williams, B.K. and K. Titus. 1988. Assessment of sampling stability in ecological applications of discriminant analysis. Ecology 69: 1275-1285. Wroble, J. 1990. Seasonal movements and spawning of adult summer steelhead trout (Salmo gairdneri) in the North Umpqua River and Steamboat Creek basin. USD1 Forest Services, Umpqua National Forest and North Umpqua Ranger District, 86 p. Wroble, J.J. and T.D. Roelof. 1985. Seasonal movements of adult steelhead in Steamboat and Canton Creeks, North Umpqua River, Oregon. A report presented to North Umpqua Ranger District, Umpqua National Forest, Oregon Department of Fish and Wildlife and Bureau of Inland Management, 41 p. Zar, J.H. 1984. Biostatistical analysis, Prentice-Hall, Inc, New Jersey, 718 p. APPENDICES APPENDIX A C C C I-. E 0-0.19 0.4-0.bY 0.2-0.39 0.8-0.99 0.6-0.79 1.2-1.39 2-2.19 1.6-1.79 1.4-1.59 1-1.19 1.8-1.99 Mean depth (m) Pools without fish Pools with fish Figure A.1: Number of fish observed in pools during the systematic survey in Steamboat Creek (August 1991). Vi V2 V2 2 } LAhocatebovi 4 41 5 5 6 6 7 7 8 8 9 9 10 10 V3 I Allocate to V2 Introduc.e V3 Allocate to V3 10 10 } Allocate to Vi Figure A.2: Conceptual example of the dichotomous key development according to Kendall (1975). See text for further explanations. (_.._____J___.. IA flfll' - "' 8%) tallow pools (18.4%) Riffles (33.9%) Glides (39.7%) Figure A.3: Proportion of habitat types in Steamboat Creek observed in the systematic survey during 1991. APPENDIX B 93 Table B.1: Percentages of bank riparian forest in pools USED and NOT USED by adult summer steelhead in Steamboat Creek, as utilized in the development of the dichotomous key. Dashed lines defines cut-off values. USED NOT USED 0 0 0 0 0 0 0 5 10 10 10 20 25 30 50 50 50 60 60 70 75 75 75 75 25 25 25 50 50 50 50 50 60 60 60 60 100 Table B.2: Maximum length in pools USED and NOT USED by adult summer ste&head in Steamboat Creek, as utilized in the development of the dichotomous key. Dashed lines defines cut-Off values. USED NOT USED 13 14 17 18 20 22 22 24 24 25 27 28 - 28 30 29 30 30 32 39 40 40 43 44 50 44 44 47 58 60 62 67 69 77 95 Table B.3: Mean bottom temperature in pools USED and NOT USED by adult summer steelhead in Steamboat Creek, as utilized in the development of the dichotomous key. Dashed lines defines cut-off values. USED NOT USED 16.59 16.60 16.63 16.69 17.00 17.08 17.46 17.73 17.95 18.00 18.50 18.58 18.90 18.83 19.86 19.90 19.90 19.02 17 19.93 19.93 19.94 20.21 20.45 20.82 21.08 21.12 21.42 21.72 22.07 22.25 22.50 22.61 22.61 22.62 22.68 Table B.4: Maximum depth in pools USED and NOT USED by adult summer steelhead in Steamboat Creek, as utilized in the development of the dichotomous key. Dashed lines defines cut-off values. USED 2.50 2.75 2.75 NOT USED 1.75 2.00 2.25 2.25 2.25 2.50 2.50 2.50 2.75 2.75 2.75 2.75 2.75 2.75 2.75 3.00 3.00 3.00 3.25 3,25 3.50 3.50 3.50 3.30 3.40 3.50 3.50 3.80 4.00 4.50 4.50 4.30 4.50 5.00 97 Table B.5: Comparative analysis of physical variables in pools in Canton Creek and Steamboat Creek. X= mean; SD= standard deviation; n.s: not significative at 0.05 level; s: significative at 0.05 level; power= 1- type II error. Variable Canton Creek Steamboat Creek Difference X= 0.29 SD= 0.222 SD = 0.291 X= 0.06 SD= 0.040 = 0.06 SD 0.098 n.s (P<0.99) = 0.39 SD= 0.12 X= 0.45 SD= 0.195 n.s (P<0.39) power= 0.31 LB X= 0.12 SD= 0.083 X= 0.14 SD= 0.135 n.s (P<0.58) power= 0.08 C X= 0.14 SD= 0.098 SD= 0.048 s (P<0.004) power= 0.80 SG X= 0.05 SD= 0.034 X= 0.06 SD= 0.06 n.s (P<0.75) power= 0.17 LG X= 0.09 SD= 0.070 SD= 0.043 X= 328 SD= 182 SD= 389 X= 502 SD= 344 SD= 684 LED S BR A VOL MD MAD DR MW 5= 1.53 X= 0.31 = 0.07 = 0.05 = 565 = 856 n.s (P<0.79) power= 0.06 n.s (P<0.06) power= 0.59 n.s (P<0.07) power= 0.45 n.s (P<0.12) power= 0.35 SD= 0.369 SD= 0.518 n.s (P< 0.69) power= 0.17 5= 3.40 SD= 0.546 X= 3.15 SD= 0.789 n.s (P<0.95) power= 0.35 = 0.45 = 0.46 SD= 0.095 SD= 0.097 n.s (P<0.75) power= 0.06 = 9.79 3= 14.05 SD= 3.336 = 1.45 SD= 4.994 s (P<0.002) power= 0.70 Table B.5 (Continued) Variable LIT ML SI Canton Creek X= 0.28 SD= 0.095 X= 31.1 SD= 12.740 = 1.40 SD= 0.414 BO 5(= 7.73 SD= 0.869 TVC 5= 1.53 SD= 1.250 BT 3(= 18.75 SD= 0.300 Steamboat Creek Difference = 0.28 n.s (P<0.99) SD= 0.099 5= 37.24 SD= 18.01 n.s (P<0.32) power= 0.17 X= 1.39 SD= 0.337 n.s (P<0.99) X= 8.75 SD= 1.906 n.s (P<0.11) power= 0.37 X= 2.71 SD= 1.640 n.s (P<0.74) power= 0.50 X= 19.52 SD= 2.113 n.s (P<0.25) power= 0.19 Table B.6: Values measured in Steamboat Creek basin pools. REA: area (m2); VOL: volume (m3) ZD: mean depth (m); ZX: maximum depth (m); DR: depth ratio; MW mean width (m); LIT: littoral area proportion; PER: perimeter (m); ML; maximum length (m); SSI: shore sinuosity index; LED:Iedge cover proportion; RF: proportion of bank riparian forest; S:sand proportion; BR: bedrock proportion; LB: large boulder proportion; SB: small boulder proportion; C:cobble proportion; SG: small gravel proportion; LG: large gravel proportion; BO: bottom oxygen (mg/L); TCV: temperature coefficient of variation; BT: mean bottom water temperature (°C) (see text for further explanation). Poo' LED S BR SB LB C SQ LG 1 0,31 0.09 0.18 0.09 0.15 0.12 0.12 0.24 2 0.74 0.10 0.40 0.16 0.11 0.20 0.05 0.07 3 0.15 0.1Q 0.33 013 0.13 0.15 0.05 0.10 4 0.26 0.00 0.37 0.14 0.26 0.14 0.03 006 5 6 0.17 0.03 0.57 0.20 0.07 0.07 0.03 0.03 0.40 0.05 0.32 0.08 0.24 0.26 0,00 0.05 7 0.20 0.00 026 0.21 0.05 0.32 0.05 0,11 8 0.10 0.08 0.52 0.20 0.12 0.04 0.04 0,00 9 0.00 0.02 0.50 0.14 0.00 0.10 0.08 016 10 0.55 0.09 0.48 0.26 0.04 0.00 0.09 0.04 11 0.95 0.03 0.38 0.17 0.24 0.09 0.03 0.06 12 0.75 0.22 0.40 0.10 0.06 0.17 0.01 0.02 13 0.00 0.06 0.26 0.26 0.17 0.06 0.13 0.06 14 0.00 0.02 0.33 0.20 0.02 0.12 018 0.13 15 0.37 0.00 0.31 0.33 0.20 0.09 0.02 0.04 16 0.05 0.25 0.14 0.19 0.14 0.15 0,07 0.07 17 0.02 0.16 0.26 0.11 0.16 0.21 0,07 18 0.00 0.40 0.05 0.51 0,10 0.13 0.10 0.05 0.05 19 0.65 0.03 0.37 0.29 0.13 0.11 0.01 0.08 20 0.30 0.55 0.00 0.47 0.24 0.03 0.12 0.03 0.12 0.04 0.60 0.13 0.02 0.09 0.11 0.00 22 23 24 25 26 27 28 29 30 0.10 0.02 0.51 0.14 0.10 0.04 0,12 0.06 0.75 0.00 0.55 0.20 0.13 0.05 0.00 0.07 0.50 0.11 0.24 029 021 0.03 0.11 0.03 0.05 0.00 0.30 0.04 0.35 0.06 0.13 0.09 0.45 0.37 0.00 0.00 0.63 0.00 0.00 0.00 0.60 0.02 0.27 0.22 0.39 0.02 0.05 0.00 0.25 0.06 0.41 0.11 0.30 0.06 0.07 0.00 0.00 0.64 0.06 0.17 0.03 0.06 0.06 0.10 0.00 0.00 0.59 0.16 0,12 0.08 0.02 31 0.00 0.00 0.75 0.13 0.06 0.00 32 33 34 35 36 37 38 39 40 0.00 0.00 0.56 0.12 0.08 0.20 0.02 0.81 0.12 0.10 0.14 0.22 0.08 0.10 0.00 0.73 0.00 0.10 0.05 0.21 0.35 0.00 0.25 0.55 0.07 0.25 0.13 0.90 41 42 43 44 45 46 47 48 21 A VOL 459 1287 566 448 367 339 137 205 564 329 0.02 435 512 484 293 203 290 70 160 655 202 976 1297 443 320 279 986 326 610 1174 987 893 272 1122 510 184 230 254 404 594 639 0.00 0.06 121 0,04 0.12 0.08 168 0.02 0.03 0.00 0.00 807 0.10 0.10 0.24 0.12 0.20 0.00 0.00 0.07 867 217 0.26 0.29 0.10 0.01 0.08 0.19 0.38 0,13 0.00 0.06 0.44 0.14 0.00 0.16 0.09 0.09 0.49 0.33 0.00 0.03 0.03 0.00 735 252 1118 277 0.03 0.68 010 0.03 0.10 0.08 0.00 1431 0.25 0.03 0.70 0.16 0.00 0.03 0.03 0.05 0.70 0.04 0.64 0.07 0.01 0.11 0.03 0.10 0.00 0.08 0.67 0.08 0.10 0.05 0.03 0.00 0.37 0.04 0.60 0.02 0.04 0.02 0.11 0.17 0.70 0.00 0.32 0.32 0.20 0.08 0.00 0.08 0.00 0.00 0.60 0.08 0.16 0.08 0.08 0.00 0.00 0.27 0.59 0.14 0.00 0.00 0.00 0.00 0.55 0.18 0.37 0.18 0.08 0.12 0.08 0.02 432 1190 230 270 169 120 114 460 1161 2055 470 410 315 1297 365 738 3035 1234 1125 478 2278 796 248 444 667 1305 647 971 310 227 928 1179 364 897 333 1465 277 2275 445 1664 250 332 602 97 108 727 100 Table B.6 (Continued) MW ZO ZX OR LIT ML SI Rff 80 TCV IF 1.30 3.50 0.37 15.40 0.40 1.36 0.50 5.80 1.69 18.22 2 2.33 4.20 0.55 16.83 0.15 29 32 1.19 0.60 9.00 4.75 17.94 3 1.41 4.00 0.35 11.33 0.38 4? 135 0.50 7.00 1.08 19.43 4 1.61 3,20 0.50 11.57 0.20 1.12 0.50 7.00 2.26 18.80 5 1.87 4.00 0.47 6.14 0.28 1.53 0.00 7.80 0.91 20.50 6 1.38 2.75 0.50 8.28 0.38 25 33 35 1.23 0.50 7.80 0.90 20.50 7 1.84 3.50 0.53 7.00 0.16 10 2.50 0.20 9.20 0.79 21.60 8 1.45 2.75 0.53 8.00 0.40 1.08 0.25 6.80 0.76 21.60 9 1.39 3.40 0.41 11.40 0.30 20 58 1.16 0.20 7.70 1.13 20,10 10 1.87 2.75 0.68 7.60 0.29 0.00 8.20 0.71 20.10 1.33 3.50 0.38 16.83 0.47 1.19 0.00 8.80 0.00 22.50 12 13 14 1.30 4.30 0.30 20.31 0.25 28 58 67 1.45 11 1.27 0.60 8.80 2.49 22.62 1.17 2.50 0.47 16.40 0.27 148 0.60 11.00 0.06 19.86 1.40 2.75 0.51 10.00 0.19 1.41 0.50 11.00 0.00 19.90 15 1.68 2.75 0.61 12.67 0.42 1.57 0.00 12.00 0.00 19.90 16 1.52 2.75 0,55 22.71 0.28 12.00 1.15 19.93 1.28 2.75 0.47 10.85 0.36 132 117 0.25 17 0.10 10.50 0.71 20.45 18 1.35 3.00 0.45 8.60 0.28 1.25 0.50 1000 0.70 21.42 19 2.27 4.50 0.50 17.80 0.13 1,32 0.70 8.50 1.22 3.00 0.41 21.00 0.34 1.29 0.50 10.00 3.08 0.74 21.12 20 21 1.43 3.30 0.43 14.80 0.45 27 32 22 39 30 44 66 47 60 20 77 30 17 49 29 40 44 50 1,14 0.60 11.50 0.47 20.82 20.45 Poal 22 1.42 3.00 '0.47 18.10 0.35 23 24 2.47 5.00 0.49 14.91 0.30 1.63 2.75 0.59 16.67 0.18 25 26 27 28 29 30 1.39 2.25 0.62 10.80 0.22 1,86 4.00 0.47 4.85 0.17 2.25 3.50 0.64 7.88 0.12 1.94 4.50 0.43 10.41 0.18 1.25 2.50 0.50 13.83 0.39 1.50 2.75 0.55 12.11 0.30 1.48 0.50 7.80 0.25 22.25 1.17 0.20 8.00 2.52 19.02 1.83 0.05 8.00 0.87 21.72 1.21 0.00 8.00 0.31 22.61 1.98 0.00 7,00 0.74 22.81 1.91 0.00 8.60 1.32 21.08 2.13 0.30 7.80 1.99 18.58 1.13 0.00 10.00 0.46 18.00 1.25 0.50 9.20 5.16 17.00 31 2,38 3.80 0.63 8.68 0.19 14 2.21 0.00 9.20 1.58 18.50 32 1,44 2.50 0.58 0.15 0.25 9.00 2.50 0.54 1.13 0.60 8.20 0.00 0.30 19.00 1.34 22 69 1.14 33 7.80 13.30 34 35 36 37 38 1.50 3.50 0.43 21.66 0.34 1.39 0.50 .7.70 2.11 17.08 1.55 3.25 0.48 9.50 0.24 1.14 0.25 9.80 1.91 16.6. 1.25 3.25 0.38 17.92 0.29 1.42 0.75 9.80 2.36 16.59 1.51 2.75 0.55 10.50 0.28 1.44 0.50 9.80 2.11 18.90 1.52 3.50 0.43 0.31 1.28 0.75 6.50 3.31 16.60 39 40 1.18 2.25 0.52 24.40 14.40 1.11 0.10 8.20 3.68 22.68 1.73 4.50 0.38 23.86 0.29 1.38 0.75 1.50 5.18 19.57 41 1.15 2.50 0.48 15.08 0.44 1.39 0.60 5.50 5.03 22.07 42 43 44 1.37 2.75 0.50 19.20 0.30 1.30 0.60 7.50 0.93 18.83 1.51 0.76 9.00 0.15 1.12 0.10 8.80 5.44 20.21 1.44 2.00 3.40 0.42 11.64 0.30 40 28 44 24 43 19 60 28 62 25 24 1.35 0.00 9.60 2,65 17.95 45 46 3.46 4.50 0.77 16.00 0.12 11 2.64 6.80 0.46 17.73 0.86 1.75 0.49 10.50 0.36 11 1.18 0.50 1.00 7.50 0.27 19.93 47 48 1.15 2.25 0.51 8.75 0.50 13 1.54 0.25 7.00 0.00 19.94 1,75 3.50 0.50 13.00 0.25 30 1.27 0.75 9.60 4.09 16.69 0.40 0.38 17.46