Common Assessment Review – Standard 7
advertisement

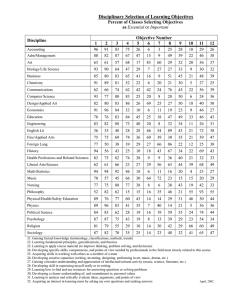
Date________________________Name_______________________________________ Per_________ Common Assessment Review – Standard 7 1. What is temperature a measure of? 2. What happens to atoms/molecules when temperature of the substance increases? Decreases? 3. As a substance warms up (no chemical reaction) where does the energy come from and where does it go? What about when it cools down? 4. Describe the flow of energy when an ice cube is placed in a glass of water. 5. Compare the speed of the atoms/molecules in each of the following phases of a substance. Solid Liquid Gas 6. What happens during an endothermic reaction? What type of energy is the reaction gaining/losing? What type of energy are the surroundings gaining/losing (usually)? 7. What happens during an endothermic reaction? What type of energy is the reaction gaining/losing? What type of energy are the surroundings gaining/losing (usually)? 8. Draw a reaction diagram for both types of reactions. Draw and label the axes and the following terms: reactants, products, activation energy (EA), enthalpy (ΔH). Date________________________Name_______________________________________ Per_________ 9. Explain how the sign of ΔH (negative or positive) relates to the energy of the reaction. 7c 10. For each of the following terms, identify if energy is absorbed or released: a. Melting b. Freezing c. Condensation d. Vaporization / Boiling 7d 11. How much heat in joules does it take to raise 5.0g of water from 65oC to 70.oC? 12. The specific heat of copper is about 0.4 joules/gram ˚C. How much heat is needed to change the temperature of a 20. g sample of copper from 40.0˚C to 70.0˚C? 13. How much heat, in joules, is required to melt 2.0 mol of ice? (The heat of fusion is 6.01 kJ/mol.) 14. Draw a heating curve and label the phase changes.