PSEUDOMONAS SYRINGAE J G Vicente and S J Roberts
advertisement

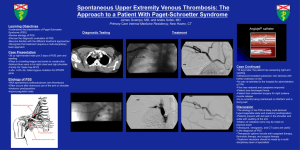
TAXONOMY AND PATHOGENICITY OF PSEUDOMONAS SYRINGAE ISOLATES FROM CHERRY (PRUNUS AVIUM) J G Vicente1 and S J Roberts2 1 Warwick-HRI, The University of Warwick, Wellesbourne, Warwick, CV35 9EF, UK. Plant Health Solutions, 20 Beauchamp Road, Warwick, CV34 5NU, UK. 2 Background ● Bacterial canker is one of the most important diseases of cherry (Prunus avium L.) and a major limitaion for timber production from wild cherry. ● Most previous studies have been on sweet cherry, with Pseudomonas syringae pv. morsprunorum (Psm) considered the primay cause in the UK and both P. syringae pv. syringae (Pss) and Psm in other other countries. ● More recently, Pss and/or intermediate forms between Psm and Pss were also found in sweet and in wild cherry. ● Our aim was to identify the pathogens associated with bacterial canker in wild cherry in England. Methods Pathogenicity ● 74 Pseudomonas syringae isolates from cherry (mostly from England 1957-2000) plus 13 others. ● Characterised by: Physiological / biochemical tests (fluorescence, colour in NSB, GATTa,); Pathogenicity; Agglutination and ELISA (three antisera); rep-PCR. Tested on micropropagated lilac (Sensation) and two wild cherry clones (Charger and 1912) and rooted lilac cv. SensaLilac Charger 1912 tion plants. Control ● Differentiated Pss and Psm isolates . ● Demonstrated Pss a range of aggressiveness amongst Pss Psm r1 isolates. +/– +e + +/– – rep-PCR Rep+Eric+Box RepEricBoxAll Rep Eric Box 100 + 95 – + + +/– 85 – + + +/– 90 10 w (4), s (5), p (1) 7 w (1), s (2), p (4) 8 w (2), s (6) 8 w (8) +/– +/– +/– 80 ––++ 9/3 105D + + 70 w 8/3 +/– 75 N Agglutinationd 65 Psm race 1 v Col. Path on Path on micropropagated in G A T Tab No. Hostsc (no.) mature Lilac Charger 1912 Spots NSB lilac y + + – – 28 w (14), s (8), + + + + – cl (1), pl (2), l (2), pr (1) 5 w (5) (+) + +/– +/– – 7 w (7) – + +/– +/– – 14 w (13), s (1) – – – – – 60 Pss Fluor.a 50 Group 55 Summary of physiological / biochemical, pathogencity and agglutination test results. Isolate Group Host Number +/+ w 7928A -/(+) w 7962 -/- w 7969 -/- w 7970A -/- w 5828 -/(+) w 7955C -/(+) w 7956 -/- w 7959 Ps -/- w 7961 Ps -/- w 5837 Pss +/+ s 7973A Ps -/- w 7932A Pss -/(+) w 7971A Ps -/- w 7927A Ps -/- w 7928C Pss +/+ w 5841 Pss -/(+) w 7919A Pss -/(+) w 5265 Ps -/- w 7929A Ps -/- w 7929D Pss (+)/+ w 7964 Pss (+)/+ w 7965A Pss (+)/+ w 7963 Pss -/(+) w 7922A Pss (+)/+ w 5264 Pss (+)/+ w 7921 Ps -/- w 7949 Pss +/+ cl SC073B Pss +/+ s 5355 Pss +/+ w 7972 Pss +/+ w 5277 Pss +/+ s 5356A Pss +/+ w 5275 Pss +/+ s 5262 Pss +/+ w 5275 Pss +/+ w 5275 Pss +/+ s 5357 Pss +/+ w 5275 Pss +/+ w 7924 Pss +/+ s 7926A Pss +/+ w 7929B Pss +/+ w 5267 Pss +/+ w 5835 Pss +/+ p 7872 Pss +/+ s 7874 Pss +/+ p 7873 Ps -/- s 2942 Pss +/+ w 5272 Pss +/+ w 8094A Pss +/+ w 8094C Pss +/+ w 8094B Pss +/+ s 7933 Pss +/+ pr 5340 Conclusions Pss +/+ l 801 Pss +/+ l 2070 my 5400 ● Bacterial canker is present throughout southern England and can be caused by either Psm or Pss. ● The GATTa tests plus the colour of growth in NSB can differentiate Psm races 1 and 2 from other P. syringae isolates. ● Serological tests or rep-PCR can be used as alternatives to the classical tests to identify Psm, but cannot replace pathogenicity for Pss. Psm race 2 Intermediate Others v B – – – – +f + +/– – g w +––– – – +/– +/– + +/– +/– – + + – w or + + – – – – +/– +/– +h yw N w +–+– 2 myr, (1), ph – – – – – – – (+) y –––– (1) a Flourescence on King’s medium B: v - variable; N - non-fluorescent, B - blue fluorescent b Gelatinase, Aesculin hydrolysis, Tyrosinae, Tartrate untilisation c Hosts: w, wild cherry; s, sweet cherry; p, plum; cl, cherry laurel; l, lilac, pr, pear; my, myrobalan; ph, peach. d Antiserum 8/3 prepared to Psm and 9/3 to Pss from wild cherry, 105D to Ps from pea. e Nine, f three, g six, and h four of these isolates produced leaf spots on plantlets of Charger and/or 1912. Total genomic DNA amplified using REP, ERIC and BOX primers. Pss Pss Ps Ps Ps Pss Pss Ps Other Other PLANT HEALTH SOLUTIONS E: s.roberts@planthealth.co.uk W: www.planthealth.co.uk Acknowledgments This work was funded by ● ● ● ● ph 5402 Psm race 2 w 5271 Ps -/- w 7920B Psm race 2 w 5845 Psm race 2 s 5260 Psm race 2 w 5831 Psm race 2 s 5260 Psm race 2 w 7967A Psm race 2 w 7968A Psm race 2 s 5260 Psm race 2 w 5836 Psm race 2 w 7958A Psm race 2 w SC214 Psm race 2 w SC217 Psm race 2 w 5268 Psm race 2 s 5253 Psm race 2 s 5261 Psm race 2 s 5252 Psm race 2 s 5260 Psm race 2 s 5250 Psm race 2 s 5255 Psm race 1 p 5281 Psm race 1 p 5299 Psm race 1 p 2928 Psm race 1 s 5244 Psm race 1 w 5833 Psm race 1 w 5269 Psm race 1 p 5300 Psm race 1 w 5270 Psm race 1 p 797 Psm race 1 s 798 Psm race 1 s 2206 Psm race 1 s 5239 Psm race 1 s 5243 Psm race 1 s 5259 Psm race 1 s 5238 Psm race 1 w 5270 Psm race 1 w 5270 Psm race 1 w 5266 Psm race 1 w 5270 Psm race 1 uniform. Psm race 2 uniform. Psm races easily differentiated. Pss highly variable. Publications Vicente, J.G. and Roberts, S.J. (2006) Discrimination of isolates of Pseudomonas syringae from sweet and wild cherry using rep-PCR. European Journal of Plant Pathology (submitted). Vicente, J.G., Alves, J.P., Russell, K. and Roberts, S.J. (2004) Identification and discrimination of Pseudomonas syringae isolates from wild cherry in England. European Journal of Plant Pathology 110, 337-351. Vicente, J.G. and Roberts, S.J. (2003) Screening wild cherry micropropagated plantlets for resistance to bacterial canker. In: Developments in Plant Pathology: Pseudomonas syringae pathovars and related pathogens ed. Iacobellis, N.S. Dordrecht: Klewer Academic Publishers. 11th International Conference on Plant Pathogenic Bacteria, Edinburgh, 10-14 July 2006 11th International Conference on Plant Pathogenic Bacteria, Edinburgh, 10-14 July 2006 Taxonomy and pathogenicity of P.syringae isolates from cherry (Prunus avium) J. G. Vicente1, and S. J. Roberts2 1 Warwick HRI, The University of Warwick, Wellesbourne, Warwick CV35 9EF, UK 2 Plant Health Solutions, 20 Beauchamp Road, Warwick CV34 5NU, UK E-mail: s.roberts@planthealth.co.uk Bacterial canker is one of the most important diseases of sweet and wild cherry (Prunus avium L.). This disease can be caused by two pathovars of Pseudomonas syringae: pv. morsprunorum (Psm) and pv. syringae (Pss). Seventy-four Pseudomonas syringae isolates from cherry and 13 isolates from other hosts were characterised by physiological, biochemical, serological and pathogenicity tests. Repetitive DNA polymerase chain reaction-based fingerprinting (rep-PCR) was also investigated as a method to distinguish pathovars, races and isolates. Physiological and biochemical tests discriminated Psm races 1 and 2 from other P. syringae isolates. Agglutination and indirectELISA tests with three different antisera showed that Psm race 1 and race 2 were very uniform and indicated high variability amongst other P. syringae isolates. However, pathogenic Pss isolates could not be distinguished from non-pathogenic isolates of P. syringae on the basis of physiological, biochemical or serological tests. Pathogenicity tests on rooted lilac plants and on micropropagated plantlets of lilac and two wild cherry clones differentiated Pss and Psm isolates and demonstrated a range of aggressiveness amongst Pss isolates. The results of rep-PCR using three sets of primers (REP, ERIC and BOX), indicated that the Pss isolates were highly variable, the two races of Psm can be easily separated and the Psm isolates are generally very uniform within each race. Serological tests or rep-PCR could be used as alternatives to the classical physiological and biochemical tests to increase the speed of detection and discrimination of isolates, but pathogenicity tests are still necessary to discriminate the pathogenic Pss isolates.