Chapter Two Joule experiment
advertisement
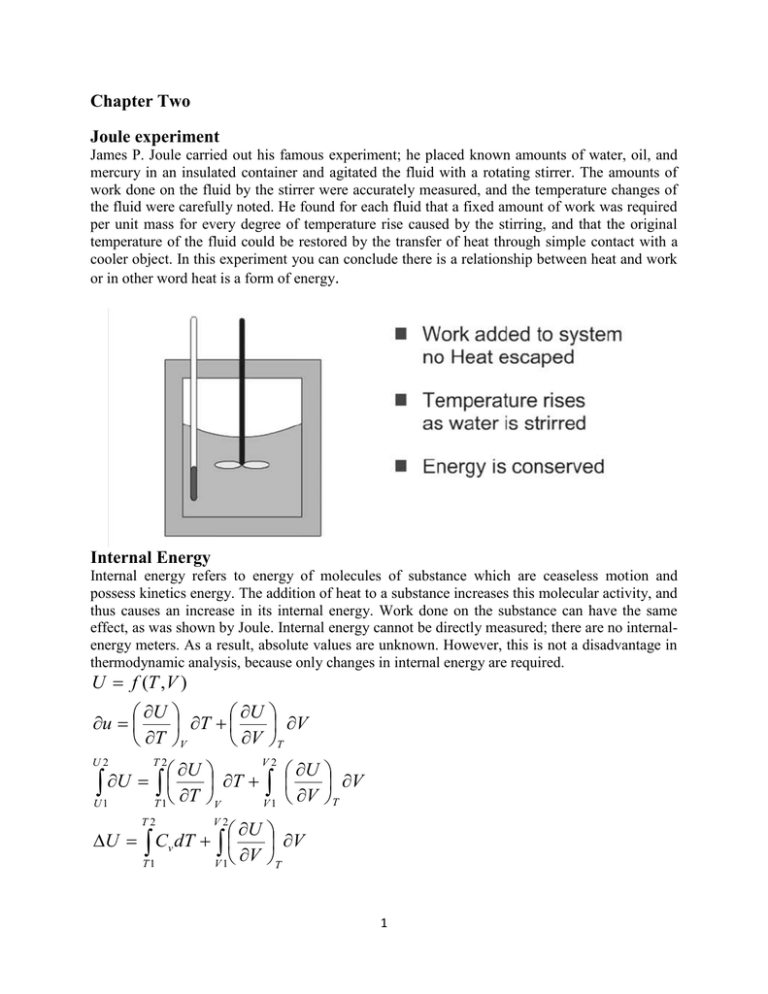
Chapter Two Joule experiment James P. Joule carried out his famous experiment; he placed known amounts of water, oil, and mercury in an insulated container and agitated the fluid with a rotating stirrer. The amounts of work done on the fluid by the stirrer were accurately measured, and the temperature changes of the fluid were carefully noted. He found for each fluid that a fixed amount of work was required per unit mass for every degree of temperature rise caused by the stirring, and that the original temperature of the fluid could be restored by the transfer of heat through simple contact with a cooler object. In this experiment you can conclude there is a relationship between heat and work or in other word heat is a form of energy. Internal Energy Internal energy refers to energy of molecules of substance which are ceaseless motion and possess kinetics energy. The addition of heat to a substance increases this molecular activity, and thus causes an increase in its internal energy. Work done on the substance can have the same effect, as was shown by Joule. Internal energy cannot be directly measured; there are no internalenergy meters. As a result, absolute values are unknown. However, this is not a disadvantage in thermodynamic analysis, because only changes in internal energy are required. U f (T ,V ) U U u T V T V V T U U U T U 1 T 1 T V 1 V T V V U2 T2 V2 U U Cv dT V V T T1 V 1 T2 V2 1 In case of case ideal gas, ( U )T 0 , and this lead to : V T2 U Cv dT T1 Internal Energy Motion & bonding of molecules Kinetic energy of molecules Potential energy of molecules Bonding energy Important factors influencing Temperature State of aggregation ; solid liquid and gas Chemical composition System: the part in which the process occurs or the part of the universe that we choose to study .The system may be of any size depending on the application, and its boundaries may be real or imaginary, rigid or flexible. Surroundings: The rest of the universe Boundary: The surface dividing the system from the surroundings 2 Classification of thermodynamic system Open: mass and energy can transfer between the system and surroundings Closed :energy can transfer between system and surroundings, but NOT mass Isolated: neither mass nor energy can transfer between the system and surroundings. Property of system All quantities which identify the state of a system like volume, temperature pressure etc. Extensive property: the properties of a system whose value for the entire system is equal to the sum of their values for the individual parts of system, these properties relates to the quantity of material involved like (volume, mass, all kinds of energy). Intensive property: the property of system whose value for entire system is not equal to the sum of their values for the individual parts of the system .these properties does not depend on the mass of the system. Path function and state function State function: the properties which does not depend on the past history of the substance nor the path it followed in reaching a given state like internal energy, enthalpy, entropy, temperature, pressure. Path function: the properties which opposite to state function i.e. the properties depend on path like work and heat. Path function Depend on path State function Depend on initial and final conditions They cannot represent on graph by points They can represented by points on graph but rather by area Have values only when there is change in Always have a values state Integration gives finite value Integration gives finite change T2 dw W dT T 2 dQ Q T1 3 T1 Firs Law of Thermodynamics Although energy assumes many forms, the total quantity of energy is constant, and when energy disappears in one form it appears simultaneously in other form. Energy cannot be created or destroyed .you can only change it from one form to another, or you can only add it to the system from the surrounding. The first law applies to the system and surroundings, and not to the system alone. In its most basic form, the first law requires: ∆ (energy of system) + ∆ (energy of surroundings) = 0 The system may change in its internal energy, in its potential or kinetic energy, and in the potential or kinetic energy of its finite parts. Since attention is focused on the system, the nature of energy changes in the surroundings is not of interest. ∆ (Energy of the surroundings) = ± Q ± W ∆ (Energy of the system) = ∆U+ ∆Ek +∆Ep First law for closed system (non-flow system) ∆ (Energy of the surroundings) + ∆ (Energy of the system) = 0 ∆U + ∆Ek +∆Ep = Q – W If ∆Ek and ∆Ep are negligible ∆U = Q – W In word the last equation states that the total energy change of the system is equal the heat added to the system minus the work done by the system. 4









