Thermochemistry Notes
advertisement

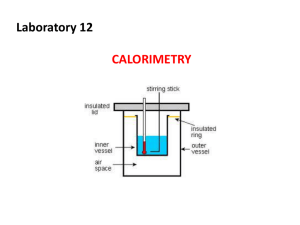
What does temperature measure? What does pressure measure? Thermochemistry Heat changes that occur during a chemical reaction Energy- the capacity for doing work or supplying heat Energy is weightless, colorless, and odorless Energy that is stored in the bonds of chemical substances is called chemical potential energy. (Example: gasoline) What is Work and Heat? Work- when a force is used to move an object Heat- energy that is transferred from one object to another Through a temperature change Neither senses nor instruments can detect heat; only changes in heat can be detected Heat flows from warm to cold until equilibrium is reached Key terms for describing the flow of energy System- part of the universe you are interested in Surroundings Surroundingseverything else in the universe System Universe- the system and the surroundings The Universe is Everything Endothermic When heat flows into a system from the surroundings the process is called endothermic +q Exothermic When heat flows out of a system into the surroundings the process is called exothermic -q What is a calorie? The quantity of heat required to raise one (1) gram of water one (1) C The unit that we will be using that is the SI system of measurement for heat is the joule 1 joule = 0.2390 calories 1 calorie = 4.184 joules Heat Capacity The amount of heat needed to raise the temperature of substance 1 C Heat capacity depends on the composition and the mass of the substance Specific Heat Capacity The amount of heat it takes to raise 1 gram of a substance 1 C We can determine the heat capacity using the equation C = Specific Heat (J/g C or cal/g C) q = heat (J or cal) m = mass (g) T = Tfinal – Tinitial (C) q Cp m T Example When 400 J of heat is added to 4.0 grams of olive oil at 20C, the temperature increases to 60C. What is the specific heat of olive oil? C=? q = 400 J m = 4.0 g T = Tfinal – Tinitial = 60C – 20C = 40 C q C m T 400 J C 4.0 g 40C J C 2.5 g C Another Example How much heat is required to raise the temperature of 200.0 g of mercury 50 C? The specific heat of mercury is 0.15 J/g C. C = 0.15 J/g C q=? m = 200.0 g T = 50 C q C m T q C m T J q 0.15 200.0 g 50C g C q 1500 J