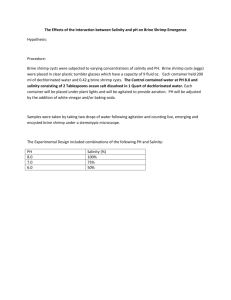
Organocatalytic Direct Michael Reaction of Ketones and Aldehydes with β Nobuyuki Mase,
advertisement

Published on Web 03/24/2006 Organocatalytic Direct Michael Reaction of Ketones and Aldehydes with β-Nitrostyrene in Brine Nobuyuki Mase,† Kaori Watanabe,† Hidemi Yoda,† Kunihiko Takabe,*,† Fujie Tanaka,‡ and Carlos F. Barbas III‡ Department of Molecular Science, Faculty of Engineering, Shizuoka UniVersity, 3-5-1 Johoku, Hamamatsu 432-8561, Japan, and The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 Received January 17, 2006; E-mail: tcktaka@ipc.shizuoka.ac.jp In 1954, Gilbert Stork showed that enamines could be used in the alkylation and acylation of aldehydes and ketones,1 and in the years since then, enamines have been intensively studied in organic synthesis.2 Recently, small chiral amines have become attractive and powerful catalysts for C-C bond forming reactions.3 The reaction, in general, is carried out by stirring a carbonyl compound 1, an amine 2, and an electrophile in conventional organic solvents, such as DMSO, DMF, or CHCl3, in a one-pot operation (eq 1). Removal of water is not required for the formation of enamine intermediates that proceed to directly react with an electrophile. While the one-pot procedure is convenient, toxic, flammable, and volatile, organic solvent is often required in these reactions. In our recent studies, we reported that a designed, small organic molecule with appropriate hydrophobic groups catalyzes enantioselective, direct aldol reactions in water (eq 2).4 Our artificial organocatalyst mimicked aldolase antibodies that act on hydrophobic organic reactants in water. Aggregation of the organocatalyst and the reactants reduces contact with bulk water as the reaction proceeds. Consequently, the outcome of the aldol reaction was similar to that performed in organic solvents. To further develop our catalyst system, we evaluated enamine-based, organocatalytic direct asymmetric Michael reactions5 of ketones and aldehydes with β-nitrostyrenes in bulk water and/or brine without using any organic cosolvent. We found that yields in brine, including seawater, the most readily available aqueous media in the world, were superior to those in water. Michael reactions of cyclohexanone (5a) with β-nitrostyrene (6a) were performed in water, as shown in Table 1. L-Proline (8) catalyzes the Michael reaction in DMSO in good yield;5 however, our first attempt in water gave no product (Table 1, entries 1 and 2). Low conversions and yields were also observed using L-prolinol (9) (entry 3). Diamine catalyst 10a improved the reactivity as well as the enantioselectivity, but resulted in low yield (entries 4 and 5). Diamine catalyst 10b, bearing a hydrophobic alkyl group with TFA, was the best catalyst of those tested in the asymmetric aldol reaction in water;4 here, it gave Michael product 7a in moderate † ‡ Shizuoka University. The Scripps Research Institute. 4966 9 J. AM. CHEM. SOC. 2006, 128, 4966-4967 Table 1. Catalyst Screening in the Direct Asymmetric Michael Reaction of Cyclohexanone (5a) with β-Nitrostyrene (6a)a entry catalyst solvent conv. (%) yield (%)b syn:antic ee (%)d 1e 8 8 9 10a 10a/TFA 10a/TFA 10b 10b 10b/TFA 10b/TFA 10b/TFA 10b/TFA 10b/TFA DMSO H2O H2O H2O H2O brine H2O brine DMSO H2O brine seawater brine 94 0 (88)f 2 (5)f 16 13 49 9 (19)f 19 76 (20)f 54 93 89 79 >95:<5 23 0 58 >99 83 >99 51 31 79 >99 >99 >99 >99 82:18 92:8 94:6 91:9 95:5 92:8 95:5 95:5 95:5 96:4 96:4 61 82 84 85 91 89 89 89 89 91 91 2 3 4 5 6 7 8 9 10 11 12 13g a Conditions: amine catalyst (0.05 mmol), additive (if used, 0.05 mmol), 5a (1.0 mmol), and 6a (0.5 mmol) in water and/or brine (0.5 mL) at 25 °C for 24 h with vigorous stirring. b Isolated yield. c Determined by 1H NMR of the crude product. d Determined by chiral-phase HPLC analysis for synproduct. e See ref 5. f Recovered yield of 6a is shown in parentheses. g Donor 5a (0.5 mmol, 1.0 equiv) was used. yield (entry 10). Despite high conversion in water, we could not obtain the Michael product in good yield because a powdery and insoluble solid side product was formed. When 6a and 10a (0.1 equiv) were stirred in water and a small amount of toluene in the absence of 5a, a quantitative amount of polymerization products of 6a was obtained as a solid. Other studies have indicated that amine catalysts behave as initiators of polymerization.6 We hypothesized that, if the anion intermediate derived from the addition of the amine catalyst to 6a could be stabilized, polymerization should be inhibited and the chemical yield should be improved. We found that the best results were obtained when brine was used as a solvent. Brine provided excellent yield and a high level of diastereo- and enantioselection (entry 11). Yield and enantioselectivity were essentially the same in seawater taken directly from the Pacific Ocean (entry 12). In these electrolyterich aqueous solutions, the anion intermediate is readily complexed by metal cations, and this ionic complexation presumably reduces polymer propagation responsible for the side product. Addition of TFA to the reaction in brine improved chemical yields by acceleration of enamine formation (entry 8 vs 11).7 The diamine 10b5h bearing dodecyl alkyl chains is a more suitable catalyst for the Michael reaction in brine than is the diamine 10a 10.1021/ja060338e CCC: $33.50 © 2006 American Chemical Society COMMUNICATIONS Table 2. Diamine 10b/TFA-Catalyzed Michael Reactions of 5a with Various β-Nitrostyrene 6 in Brinea entry Ar 1 2 3 4 5 Ph 4-MeOC6H4 2-furyl 2-naphthyl 3-NO2C6H4 product 7a 7b 7c 7d 7e time (h) yield (%)b syn:antic ee (%)d 12 20 24 48 96 93 98 94 99 57 95:5 96:4 96:4 98:2 74:26 89 83 86 97 83 a Conditions: amine catalyst 10b (0.05 mmol), TFA (0.05 mmol), 5a (1.0 mmol), and 6a (0.5 mmol) in brine (0.5 mL) at 25 °C with vigorous stirring. b Isolated yield. c Determined by 1H NMR of the crude product. d Determined by chiral-phase HPLC analysis for syn-product. Table 3. Diamine 10b/TFA-Catalyzed Michael Reactions of Various Ketones and Aldehydes 5 with 6a in Brinea entry 1 2 3f 4g 5 6 7 8 R1 R2 -(CH2)4-(CH2)3-CH2CH2SCH2Me H H H H Me H Me H Me R3 H H H H Et Me Et Pr product 7a 7f 7g 7h 7i 7j 7k 7l time (h) yield (%)b 12 96 96 24 24 30 72 96 93 75 67 87 99 76 74 97 syn:antic 95:5 77:23 97:3 65:35 59:41 61:39 ee (%)d,e 89 80 87 32 38 76 74 64 a Conditions: amine catalyst 10b (0.05 mmol), TFA (0.05 mmol), 5 (1.0 mmol), and 6a (0.5 mmol) in brine (0.5 mL) at 25 °C with vigorous stirring. b Isolated yield. c Determined by 1H NMR of the crude product. d Determined by chiral-phase HPLC analysis for syn-product. e (2S)-synIsomers were obtained as a major product in entries 1-4, and (2R)-synisomers were obtained in entries 5-8. f Toluene (100 µL) was added. g Donor (10 equiv) was used. in terms of chemical yield (entries 6 and 11). The reaction rate in brine is also faster than that in DMSO (entry 9 vs 11). Generally, an excess amount of a ketone donor (10-20 equiv) is employed in conventional organic solvents;5 however, here, an equal amount of 5a was sufficient to complete the Michael reaction in brine in 79% yield with 91% ee (entry 13). We propose that brine improves the chemical yield and stereoselectivity compared to reaction in organic solvent or water through the following mechanism:8,9 A liquid organic donor 5 assembles in brine due to hydrophobic interactions. Diamine 10b/TFA catalyst with its hydrophobic tails and the acceptor 6 dissolve in the liquid organic phase. Aggregation of the organic molecules excludes water from the organic phase and drives the equilibrium toward enamine formation. This is further facilitated by a salting-out effect. Michael reactions occur quickly in this highly concentrated organic phase through a transition state similar to that observed in organic solvents. Catalyst 10b/TFA was chosen for the further study of asymmetric Michael reactions with various nitroalkene acceptors (Table 2). The reaction appears quite general with respect to the nature of the aromatic Michael acceptors. Generally, excellent yields and high stereoselectivities, ranging from 83 to 97% ee, were observed. Next we probed the scope of the reaction with a variety of ketones and aldehydes (Table 3). Cyclic ketones were suitable as Michael donors, providing high diastereoselectivity as well as enantioselectivity (entries 1-3). Addition of a small amount of toluene (100 µL) was sufficient to facilitate the Michael reaction of the thiopyran 5g with 6a, although both substrates are solid and insoluble in water (entry 3). Michael products 7j-l bearing an allcarbon quaternary stereocenter5f were obtained with enantioselectivities similar to or slightly lower than those obtained in reactions with DMSO solvent (entries 6-8). These experiments indicate that our catalyst system in brine should be broadly applicable to the synthesis of γ-nitro carbonyl compounds. To study a multigram-scale synthesis, TFA (0.1 equiv) and 10b (0.1 equiv) were stirred with 6a (10 mmol, 1.51 g) and 5a (1.2 equiv) in brine (10 mL) at 25 °C. Solid Michael product 7a gradually formed. The mixture was stirred for 24 h, and the brine was removed to give the crude product (99% conversion, syn:anti ) 97:3, 89% ee) that was then purified by recrystallization from ethyl acetate to afford 7a (1.80 g, 73%, syn-isomer, >99% ee). No extraction, washing, nor chromatography were needed to obtain the product with excellent purity; therefore, this procedure may afford great advantage to pharmaceutical and industrial processes. The major product 7a generated from the 10b/TFA-catalyzed reaction had (1′R,2S) absolute stereochemistry.5 The absolute stereochemical results can be explained by related transition state models previously discussed for diamine 10a/acid-catalyzed Michael reactions in organic solvent.5f,g In summary, we have developed a catalytic direct asymmetric Michael reaction that can be performed in brine without addition of organic solvents. The diamine 10b/TFA bifunctional catalyst system demonstrated excellent reactivity, diastereoselectivity, and enantioselectivity in brine. Further studies focusing on the full scope of this catalyst in aqueous media and related systems are currently under investigation and will be reported in due course. Acknowledgment. This study was supported in part by a Grantin-Aid (No. 16550032) from Scientific Research from the Japan Society for the Promotion of Science and The Skaggs Institute for Chemical Biology. Supporting Information Available: Experimental procedures and HPLC data. This material is available free of charge via the Internet at http://pubs.acs.org. References (1) Stork, G.; Terrell, R.; Szmuszkovicz, J. J. Am. Chem. Soc. 1954, 76, 2029. (2) Rappoport, Z. The Chemistry of Enamines; Wiley-VCH: Weinheim, Germany, 1994. (3) (a) Acc. Chem. Res. 2004, 37 (8), special issue on organocatalysis. (b) Dalko, P. I.; Moisan, L. Angew Chem., Int. Ed. 2004, 43, 5138. (4) Mase, N.; Nakai, Y.; Ohara, N.; Yoda, H.; Takabe, K.; Tanaka, F.; Barbas, C. F., III. J. Am. Chem. Soc. 2006, 128, 734. (5) Enamine-based Michael reaction of carbonyl compounds with β-nitrostyrenes in organic solvent. (a) Sakthivel, K.; Notz, W.; Bui, T.; Barbas, C. F., III. J. Am. Chem. Soc. 2001, 123, 5260. (b) Betancort, J. M.; Sakthivel, K.; Thayumanavan, R.; Barbas, C. F., III. Tetrahedron Lett. 2001, 42, 4441. (c) Betancort, J. M.; Barbas, C. F., III. Org. Lett. 2001, 3, 3737. (d) Alexakis, A.; Andrey, O. Org. Lett. 2002, 4, 3611. (e) Enders, D.; Seki, A. Synlett 2002, 26. (f) Mase, N.; Thayumanavan, R.; Tanaka, F.; Barbas, C. F., III. Org. Lett. 2004, 6, 2527. (g) Ishii, T.; Fujioka, S.; Sekiguchi, Y.; Kotsuki, H. J. Am. Chem. Soc. 2004, 126, 9558. (h) Betancort, J. M.; Sakthivel, K.; Thayumanavan, R.; Tanaka, F.; Barbas, C. F., III. Synthesis 2004, 1509. (i) Cobb, A. J. A.; Longbottom, D. A.; Shaw, D. M.; Ley, S. V. Chem. Commun. 2004, 1808. (j) Wang, W.; Wang, J.; Li, H. Angew. Chem., Int. Ed. 2005, 44, 1369. (k) Hayashi, Y.; Gotoh, H.; Hayashi, T.; Shoji, M. Angew. Chem., Int. Ed. 2005, 44, 4212. (l) Terakado, D.; Takano, M.; Oriyama, T. Chem. Lett. 2005, 34, 962. (m) Xu, Y.; Cordova, A. Chem. Commun. 2006, 460. (n) Kotrusz, P.; Toma, S.; Schmalz, H.-G.; Adler, A. Eur. J. Org. Chem. 2004, 1577. (6) Carter, M. E.; Nash, J. L., Jr.; Drueke, J. W., Jr.; Schwietert, J. W.; Butler, G. B. J. Polym. Sci., Polym. Chem. Ed. 1978, 16, 937 and references therein. (7) Mase, N.; Tanaka, F.; Barbas, C. F., III. Org. Lett. 2003, 5, 4369. (8) Li, C.-J.; Chan, T.-H. Organic Reactions in Aqueous Media; WileyVCH: Weinheim, Germany, 1997. (9) (a) Breslow, R. Acc. Chem. Res. 1991, 24, 159. (b) Mase, N.; Ohno, T.; Morimoto, H.; Nitta, F.; Yoda, H.; Takabe, K. Tetrahedron Lett. 2005, 46, 3213. JA060338E J. AM. CHEM. SOC. 9 VOL. 128, NO. 15, 2006 4967 Supporting Information Organocatalytic direct Michael reaction of ketones and aldehydes with β-nitrostyrene in brine Nobuyuki Mase,† Kaori Watanabe,† Hidemi Yoda,† Kunihiko Takabe,*,† Fujie Tanaka,‡ Carlos F. Barbas III‡ Department of Molecular Science, Faculty of Engineering, Shizuoka University, 3-5-1 Johoku, Hamamatsu 432-8561, Japan and The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 tcktaka@ipc.shizuoka.ac.jp † Shizuoka University, ‡The Scripps Research Institute General. Chemicals and solvents were either purchased from commercial suppliers or purified by standard techniques. For thin-layer chromatography (TLC), silica gel plates (Merck 60 F254) were used and compounds were visualized by irradiation with UV light and/or by treatment with a solution of phosphomolybdic acid in ethanol followed by heating. Flash column chromatography was performed using KANTO silica gel 60N (particle size 63-210 μm). 1H NMR and 13C NMR spectra were recorded on JEOL JNM-AL spectrometer at ambient temperature. Chemical shifts are given in δ relative to tetramethylsilane (TMS), the coupling constants J are given in Hz. The spectra were recorded in CDCl3 as solvent at ambient temperature. TMS served as internal standard (δ = 0 ppm) for 1H NMR and CDCl3 was used as internal standard (δ = 77.0) for 13C NMR. Infrared spectra were recorded on a SHIMADZU FTIR-8200A spectrometer. Mass spectra were recorded on a SHIMADZU GCMS-QP5050 spectrometer. HPLC was carried out using a SHIMADZU LC-10AD intelligent pump, SPD-10A UV detector, and C-R8A integrator. All Michael reactions were carried out under an atmosphere of air in a closed system. Organic substrates cyclohexanone (5a, 108-94-1), cyclopentanone (5f, 120-92-3), tetrahydrothiopyran-4-one (5g, 1072-72-6), acetone (5h, 67-64-1), n-butyraldehyde (5i, 123-72-8), isobutylaldehyde (5j, 78-84-2), 2-methylbutyraldehyde (5k, 96-17-3), 2-methylvaleraldehyde (5l, 123-15-9), trans-β-nitrostyrene (6a, 5153-67-3), L-proline (8, 147-85-3), L-prolinol (9, 23356-96-9), and (S)-1-(2-pyrrolidinylmethyl)pyrrolidine (10a, 51207-66-0) were obtained from commercial sources and were used without any purification. Michael acceptors, such as (E)-1-methoxy-4-(2-nitrovinyl)benzene (6b, 5576-97-6), (E)-2-(2-nitrovinyl)furan (6c, 32782-45-9), (E)-2-(2-nitrovinyl)naphthalene (6d, 37629-37-1), and (E)-1-nitro-3-(2-nitrovinyl)benzene (6e, 34441-47-9) were prepared by the methods previously described.1 Catalyst 10b was prepared by the methods previously described.2 Michael adducts 7 are all known compounds. Seawater was taken from Pacific Ocean at Nakatajima Dune in Hamamatsu city and used without any purification. A representative procedure for the Michael reaction using the diamine/acid bifunctional catalyst in brine was the following procedure for the reaction of cyclohexanone (5a) with trans-β-nitrostyrene (6a) in brine using the diamine 10b/TFA bifunctional catalyst: To a mixture of diamine 10b (19.0 mg, 0.05 mmol) in brine (0.5 mL) trifluoroacetic acid (3.9 μL, 0.05 mmol) was added at 25 °C under air in a closed system. The reaction mixture was stirred for 3 min, then cyclohexanone (5a, 104 μL, 1.0 mmol) and trans-β-nitrostyrene (6a, 75.3 mg, 0.5 mmol) were added. The reaction mixture was stirred for 24 h. Brine was removed by decantation. If extraction was needed, brine phase was extracted with ethyl acetate (3 x 1 mL) and organic extracts were dried over Na2SO4. Diastereoselectivity and conversion were determined by 1H NMR analysis of the crude Michael product after short column chromatography purification (SiO2, 1 g) to remove the catalyst. Purification by flash column chromatography (silica gel, hexane/AcOEt) gave the Michael product 7a as a colorless solid. The enantiomeric excess (ee) of 7a was determined by chiral HPLC analysis. References 1. Andrey, O.; Alexakis, A.; Bernardinelli, G. Org. Lett. 2003, 5 (14), 2559-2561. 2. (a) Betancort, J. M.; Sakthivel, K.; Thayumanavan, R.; Tanaka, F.; Barbas, C. F., III Synthesis 2004, (9), 1509-1521. (b) Betancort, J. M.; Barbas, C. F., III Org. Lett. 2001, 3 (23), 3737-3740. (c) Asami, M. Bull. Chem. Soc. Jpn. 1990, 63 (3), 721-727. S1 1 No. 7a structure CAS No. O NO2 H NMR (CDCl3, -CHAr, δ)a HPLCb Flow rate (mL/min) syn (min) anti (min) 80:20 0.5 19.981 (2R, 1’S) 28.557 (2S, 1’R) - AD-H 80:20 0.5 18.467 (2R, 1’S) 22.583 (2S, 1’R) 20.333 23.408 4.18 AD-H 95:5 0.5 30.300 (2S, 1’R) 37.617 (2R, 1’S) 22.183 36.150 3.95 4.18 AS-H 50:50 0.7 8.779 (2R, 1’S) 14.907 (2S, 1’R) 12.066 12.381 3.95 4.22 AD-H 90:10 0.5 42.650 (2R, 1’S) 51.667 (2S, 1’R) 48.350 62.883 column Eluentc syn anti (S,S)-84025-87-6 (R,R)-154568-74-8 (S,R)-84025-83-2 (R,S)-84025-84-3 relative-(S,S)-51262-17-0 relative-(R,S)-51262-18-1 racemic-4591-64-4 3.78 4.02 AS-H (S,R)-233257-09-5 relative-(S,S)-99391-23-8 relative-(R,S)-67861-09-0 racemic-342819-96-9 3.71 3.91 (S,S)-256338-66-6 3.97 (S,R)-748788-76-3 (S,R)-83976-47-0 OMe 7b O NO2 O 7c 7d O NO2 O NO2 NO2 7e O NO2 S2 O 7f NO2 O 7g NO2 (R,R)-359876-15-6 (S,R)-359876-14-5 (R,S)-475294-96-3 relative-(S,S)-97954-71-7 relative-(R,S)-67861-25-0 racemic-4591-65-5 3.70 3.83 AS-H 80:20 0.5 21.858 (2R, 1’S) 30.525 (2S, 1’R) 19.575 23.492 (S,S)-360062-52-8 3.98 4.15 AS-H 50:50 0.5 16.918 (2R, 1’S) 20.760 (2S, 1’R) - AS-H 80:20 0.5 S 7h O NO2 (S)-475589-23-2 (R)-361204-86-6 chiral-(+)-142232-08-4 chiral-(-)-122348-55-4 racemic-1574-86-3 4.00 1 No. structure CAS No. O 7i NO2 H O 7j NO2 H O 7k H NO2 (S,R)-475294-88-3 (R,S)-384354-59-0 relative-(R,S)-697745-79-2 racemic-802552-10-9 H NMR (CDCl3, -CHO, δ)a (R,R)-748807-19-4 HPLCb Flow rate (mL/min) syn (min) anti (min) 99:1 1.0 23.733 (2R, 3R) 28.233 (2S, 3S) 25.567 27.017 AS-H 90:10 0.5 AD-H 97:3 0.5 syn anti column Eluentc 9.69 9.46 AD-H (R)-748807-16-1 racemic-93086-29-4 9.54 9.52 9.51 S3 27.561 (R) 36.247 (S) 22.500 (S) 23.467 (R) 23.600 (2R, 3R) 26.133 (2S, 3S) 25.358 27.142 O 7l H NO2 (R,R)-748807-20-7 9.52 9.51 a OD-H Diastereoselectivities were determined by peak integration of -CHAr for 7a-7h and of -CHO for 7i-7l. eluent. S4 95:5 b λ = 254 nm. 1.0 c 27.850 (2R, 3R) 40.942 (2S, 3S) 21.650 43.517 Hexane and 2-propanol were used as an