W. Clark Still Jonathan Lockner Baran GM 2010-07-10
advertisement
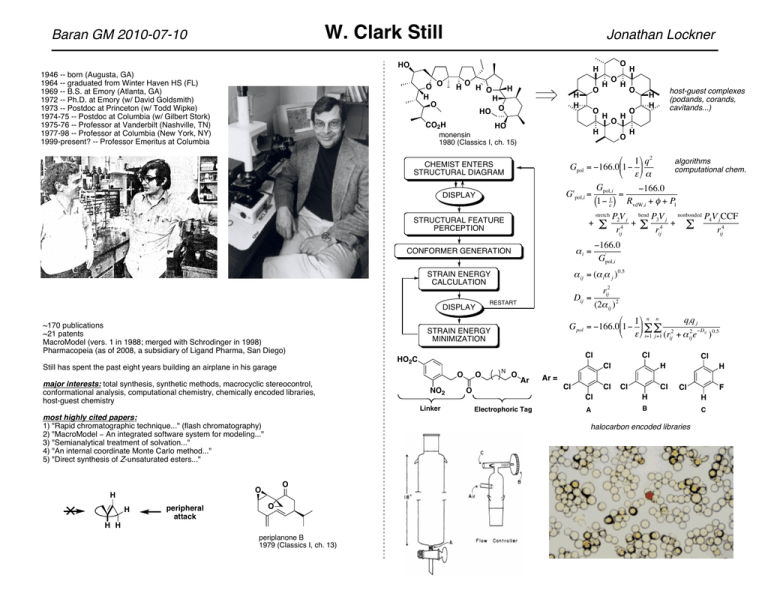
W. Clark Still Baran GM 2010-07-10 Jonathan Lockner HO O O H O H O H O H HO O HO +i = O - NO2 O O H P2V j bend P3V j nonbonded P4V j CCF + - 4 + rij4 rij rij4 !166.0 ' Gpol,i rij2 (2+ ij ) 2 % 1( n n qiq j G pol = !166.0'1! * - - 2 & $ ) i=1 j=1 (rij + + ij2 e!Dij ) 0.5 Cl O host-guest complexes (podands, corands, cavitands...) H H N Cl Cl O Ar# Ar = O Electrophoric Tag Cl Cl Cl H Cl Cl H Cl O O H H periplanone B 1979 (Classics I, ch. 13) F Cl H H A B C halocarbon encoded libraries O peripheral attack RESTART STRAIN ENERGY MINIMIZATION Linker H Dij = HO2C most highly cited papers: 1) "Rapid chromatographic technique..." (flash chromatography) 2) "MacroModel ! An integrated software system for modeling..." 3) "Semianalytical treatment of solvation..." 4) "An internal coordinate Monte Carlo method..." 5) "Direct synthesis of Z-unsaturated esters..." H O + ij = (+ i+ j ) 0.5 STRAIN ENERGY CALCULATION major interests: total synthesis, synthetic methods, macrocyclic stereocontrol, conformational analysis, computational chemistry, chemically encoded libraries, host-guest chemistry O stretch + CONFORMER GENERATION ~170 publications ~21 patents MacroModel (vers. 1 in 1988; merged with Schrodinger in 1998) Pharmacopeia (as of 2008, a subsidiary of Ligand Pharma, San Diego) H H H % 1( q2 algorithms Gpol = !166.0'1! * computational chem. & $) + G !166.0 G'pol,i = pol,i1 = (1! $ ) RvdW,i + , + P1 STRUCTURAL FEATURE PERCEPTION DISPLAY H O H DISPLAY " O monensin 1980 (Classics I, ch. 15) CHEMIST ENTERS STRUCTURAL DIAGRAM H O H H H CO2H Still has spent the past eight years building an airplane in his garage O H 1946 -- born (Augusta, GA) 1964 -- graduated from Winter Haven HS (FL) 1969 -- B.S. at Emory (Atlanta, GA) 1972 -- Ph.D. at Emory (w/ David Goldsmith) 1973 -- Postdoc at Princeton (w/ Todd Wipke) 1974-75 -- Postdoc at Columbia (w/ Gilbert Stork) 1975-76 -- Professor at Vanderbilt (Nashville, TN) 1977-98 -- Professor at Columbia (New York, NY) 1999-present? -- Professor Emeritus at Columbia W. Clark Still Baran GM 2010-07-10 allyloxy carbanions # $ O R secBuLi OSiEt3 THF "78°C Li R' OSiEt3 RI secBuLi THF-HMPA "78°C H2O R O H OH SiMe3 Me3SiLi KH tBu Et2O Me3SnLi THF O preisocalamendiol 1977 SiMe3 tBu note Z,E stereochem but Me3SiLi N.R. O a) Me3SnLi b) Me3SiCl "we felt that the conformational properties of the cyclodecadienes and the chemical reactivity of organotin compounds might merge to provide a simple solution to the isomerization problem." MnO2 O OSiMe3 take-home messages: • extent of 1,4-addition ! to solvent basicity • results contrary to HSAB theory predictions • electron transfer at play (with Me3C and Me3Si) O -O Nu- acoragermacrone 1977 Nu Nu -O O dialkylative enone transposition Me3SiLi Me3SnLi O HO Group IV anions (C, Si, Sn) 1,2 vs 1,4 addition OH tBu frullanolide 1977 a) LDA b) HOAc O OH 1,4-axial attack (e- trans. mech) tBuLi THF HMPA O Me3Sn Et2O O O O RLi tBuLi THF HMPA O germacranes Et2O"HMPA "20°C O I 2) KI3 NaHCO3 CO2Me OH RM = MeLi"Me2CuLi ("70°C) O c) heat d) Me2SO4 N DBU 1) KOH H mech? high yield equatorial methylation of unhindered cyclohexanones Me3SiLi O O Me (91% yield) tBu tBu 2) acryloyl Cl 3) pyrrolidine OH OH RM tBu O a) LDA b) Et3SiCl 1) Li base 2) NH2NH2 H2O"MeOH O secBuLi THF-HMPA "78°C O O H OSiEt3 but O O "methylenic Claisen" 1) NaOOH R H (86%) O eudesmanes why? #-attack O OH R' R OH OH R' H3O+ R OSiEt3 $-attack Jonathan Lockner Me3Si O O 2) MeLi, Et2O 3) CrO3•2pyr 4) NaOH OH SnMe3 OLi SnMe3 1) Me3SnLi THF"NH3 iodopentane stannylation/destannylation O cf. SnBu3 1) nBuLi O O dihydrojasmone 1977 O H O Me3Sn Bu3SnCH2MOM 1) Bu3SnLi 2) MeCH(Cl)OEt O geranyl Cl O OEt 2) Li"NH 3 dendrolasin 1978 "Whereas an allyl organometallic may undergo isomerization, the carbinyl carbanion approach... assures that the geometry of the central trisubstituted double bond is unambiguously retained." W. Clark Still Baran GM 2010-07-10 "flash chromatography" 1978 -- "Still demonstrated FC at a Thursday evening seminar to the department at Columbia - he separated the individual colors in black ball point pen ink faster with FC than he could run a TLC - it was VERY well received by all" Jonathan Lockner "-chelation (5-membered chelate ring) ! Grignard reagents RMgBr TBDMSO H O Still!Wittig [2,3]-sigmatropic rearrangement CO2Et 1) KH Bu3SnCH2I BuLi steps sex attractant >95% Z TBDMSO OBn MeMgCl TBDMSO H O threo (50:1) OH H O THF OBn OAc 2) Ac2O!pyr OH O O TBDMSO erythro (50:1) OH H O THF adjacent sterocenters OBn OBn double Still!Wittig [2,3] #-chelation (6-membered chelate ring) ! organocuprates 1) KH Bu3SnCH2I BuLi OEE OH CHO 2) Ac2O!pyr 3) TsCl 4) LiAlH4 5) H3O+ OH Me2CuLi OH !78 °C O >95% Z,Z O Me threo (30:1) OH O HO muscarine 1980 OBn OBn HO O O H O OH CHO configurationally stable Sn/Li alkoxy compounds ! modest stereocontrol for polypropionate synthesis (5:1, !78 °C) (8:1, !110 °C) Me 1) Bu3SnLi BnO O OMOM O OMOM Me Me 2) MOM-Cl BnO O Me + OMOM BnO O HO O OMOM HO H B OH 1) thexyl borane Me H monensin 1980 (Classics I, ch. 15) 2) NaOOH 1) Me-M H O remote asymmetric induction (via cyclic hydroboration) a. BuLi b. Me2SO4 CHO O O H O ansa bridge of rifamycin S 1983 SnBu3 BnO OAc O H CO2H Me BnO O Me SnBu3 + 2) MOM-Cl NMe3Cl H in t.s., B-H eclipses C=C, while chain in least strained conformer 96% 8:1 dr OH remote sterocenters application: TBDMSO 1) borane (1:1, !78 °C, M = MgBr) (1:1.4, !78 °C, M = Li) HO2C OH OH steps O HO2C O 2) NaOOH 1:1 Prelog-Djerassi lactonic acid 1981 Me Me O OTHP BF4 PPh3 KHMDS THF!HMPA highly Z-selective Wittig Me BF4 AsPh3 O OTHP 83% yield (>97% Z) R H KHMDS THF!HMPA H O R Me H >50:1 trans:cis Corey!Chaykovsky using arsonium ylide W. Clark Still Baran GM 2010-07-10 germacranes (continued) H " O OR H H periplanone B 1979 (Classics I, ch. 13) Me Me H H H H Me H H O O OR steps H O H HO BzO H9e OH steps H O OH O H OH trichodermol 1980 7 O O H2C Me O H H Me O Me OR O 2% aq O H2SO4 H9a O H Grob O O O H THF 0°C 2) NaOH EtOH acetone 45°C H OH Favorski macrocyclic stereocontrol H2C NaH steps OR 8 Me EEO 1) tBuOOH Triton B EtO2C O O BzO OMs O O O peripheral attack H trichothecanes (cf. his PhD work at Emory with Goldsmith) O O Jonathan Lockner HO H H O H O O O O H O O verrucarin A 1981 O O O roridin E 1984 O H O O O O Me H H O O H HO O baccaharin B5 1984 HO O O O H O H HO "The [epoxidation] reaction... cleanly establishes four new asymmetric centers of which two appear to be controlled by the conformation of the macrocycle." RO RO HO O O H O H O 1) H+ RO 2) B!V [O] 3) NaBH4 OH O O mCPBA O OH O O (74%) O O O O O O 1) K2CO3-MeOH 2) Collins [O] HO 3) DBU 4) NaBH4 5) H2-Pd(OH)2 O O remote epoxide gives modest diast. during CrCl2 macrocyclization O O O OH O O O O OH eucannabinolide 1983 O O O steps OH OH sapon; HOAc (94%) HO asperdiol 1983 O 3 3-deoxyrosaranolide 1984 11 kinetically-controlled reactions on a 16-membered macrolide were used to establish 7 of the 9 stereocenters O O O cf. monensin O HO HO W. Clark Still Baran GM 2010-07-10 macrocyclic stereocontrol host-guest chemistry, computational chemistry, and combinatorial chemistry... Me2CuLi BF3 O 39 OH OH H steps O Moving beyond monensin (a natural podand ionophore), Still's group investigated a variety of host structural motifs. Design considerations included: ease of synthesis, incorporation of symmetry elements (to minimize conformational heterogeneity), organic solvent vs. water solubility, flexible vs. rigid binding pockets, appropriate donor-acceptor motifs, hydrophobicity/hydrophilicity... 34 39 34 O !78 °C (92%) O O O Early on, progress appears to have been slow. Computational limitations needed to be overcome by either (a) waiting for hardware to improve or (b) writing code that would get the job done in less CPU time but without significant sacrifice in quality of simulation results. So, there was plenty of coding and debugging going on in the Still lab during the 80s and 90s. most stable conformer EtMgBr CHCl3 O 43 !30 °C (69%) H 39 H O O 43 steps 34 34 OH H 39 H O 30 C30-C43 segment of palytoxin 1982 1) NBS 2) (MeO)3P DEAD OH 2) Mukaiyama dehydration O O O CO2H O O 3) PS-SnH 4) sapon. O OH H N R N N R N H thromboxane A2 1985 6 OH I CO2H HO However, at some point, there seems to have been a transition (or at least a major tilt in emphasis) from rational design to "irrational synthesis" ! the emergence of split/mix library generation, chemical encoding, fluoresence assays. One can make judicious use of both enterprises, of course. In any case, it strikes me that Still was a pioneer in all of the above... macrocyclic "chemocontrol" 1) Corey! Nicolaou Jonathan Lockner O I O N HN H O N NO2 H O2N O O2N I O O N N O O I O N OH H N thromboxane B2 A Br CO2Me Bu3SnH O O compare: CO2Me O H N O OH OH N H O O H N A Still!Gennari modification of Horner!Emmons olefination kinetic elimination prior to isomerization gives Z isomer R H KHMDS, 18-crown-6 THF, !78 °C O N H O O S N H O O S HN H N H N HN S O O O (CF3CH2)2P(O)CH2CO2Et O H EtO R Z-selective Horner!Emmons cf. Ando's reagent: (PhO)2P(O)CH2CO2Et Boc H Boc S O O N H N R peptide guest A O H N O NO2 O O B O NH H H N H N O N H O H N N H O B N H A L NH CH2 CH2 R N O O OR Cu2+ N N R' R' N W. Clark Still Baran GM 2010-07-10 Cl HO2C O NO2 Linker N O Cl Cl O Ar Cl H H Ar = Cl O Cl Cl Cl Electrophoric Tag A Cl Cl F H H B C halocarbon encoded libraries Jonathan Lockner "results suggest that designs for the most selective receptors should aspire to total conformational homogeneity, not just conformationally well-defined functionality in the binding site. It is also instructive to note that increasing the flexibility of our receptor resulted in a decrease in binding of the otherwise selectively bound guests. Thus while flexible receptors may adapt their structures to fit a guest, there is a price in binding energy as well as in selectivity which must be paid." Tet Lett 1993 Separate Beads into 3 Groups OH Step 1 A+ 1% T1 T1 O A OH OH C+ 1% T1 + 1% T2 B+ 1% T2 T2 O B T1 T2 O C Mix Beads and Separate into 3 Groups T1 Split synthesis encoded with multiple tagging molecules (T1-T4) O A T2 O B T1 T2 O C Step 2 O A T2 O B T1 T2 O C X+ 1% T3 T1 T3 T2 T3 T1 T2 T1 O A T2 O B T1 T2 O C Z+ 1% T3 + 1% T4 Y+ 1% T4 O AX T1 T4 O BX T2 T4 O CX T3 Equilibrate ligand library with host (or vice-a-versa): manually "cherry-pick" the highly colored beads T1 O AY T1 T3 O BY T2 T3 O AZ "simple, host-like molecules can bind peptides with significant sequence-selectivity. Thus the prognosis is good for creating synthetic small molecules with binding properties similar to those of natural antibodies. "combinatorial synthesis and screening offers an effective approach to many chemical problems where too little is known for a deterministic solution. It also provides an important tool for testing specific hypotheses via batteries of measurements that can be applied in single experiments and thus to test hypotheses in many different contexts. "We are now using it to find small-molecule receptors for particular peptide substrates via preparation of encoded combinatorial libraries of receptors. In these libraries, we use what we know about effective peptide receptors to design a relevant, basic receptor structure and then vary that structure combinatorially to make up for what we do not know about binding a particular substrate." Acc Chem Res 1996 T4 T1 T2 O CY T4 O BZ performing conformational searches: T4 T1 T2 O CZ T3 T4 molecular geometry is described in mathematical terms via a coordinate system: e.g. torsion angles, Cartesian coordinates, matrices of internuclear distances variations of coordinates may be systematic, random (stochastic), or a mixture thereof e.g. Monte Carlo (coined by physicists at LANL) techniques are random select a force field (AMBER*, MM2, MM3, etc.) choose a solvation model (GB/SA developed by Still et al. is industry standard) Decode: in this case, the halocarbon tags are amenable to GC analysis other problem-specific algorithms may or may not be included e.g. applying a frozen atom approximation in order to limit extent of variations run the search, interpret the results of the simulation



