Applications of Mn(III) in Organic Chemistry
advertisement

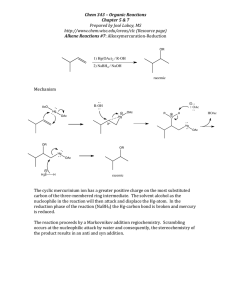
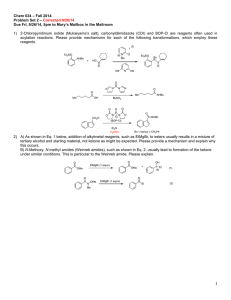
Applications of Mn(III) in Organic Chemistry Baran lab GM 2/6/2010 Contents: Oxidative radical cyclization of β-keto acids Discussion of the reaction mechanism Oxidative radical cyclization of β-keto esters Oxidative radical cyclization of 1,3-diketones Oxidative cyclization of ketones Oxidative fragmentation-cyclization Asymmetric radical cyclization Miscellaneous applications of Mn(III) salts Mn(OAc)3 This summary will not address the chemistry of Mn(III)salen complexes and Mn(III)porphyrins. For a review on the Jacobsen-Katsuki epoxidation see: Jacobsen, Catal. Asymmetric Synth. (ed. Ojima, I.), 159-202, (VCH, New York, 1993) and ref therein For a representative example in the field of Mn(III) porphyrins see Groves, J. Am. Chem. Soc., 1988, 110, 8628. Thornton, J. Chem. Soc. Chem. Comm., 1978, 62. - crystallizes as Mn3O(OAc)7 - anhydrous form does not exist - sold as Mn(OAc)2• 2H2O - insoluble in most organic solvents; soluble in hot AcOH - can be prepared in situ from Mn(OAc)2 and KMnO4 in AcOH First reports... a) Ph Reductive processes H • O MnIII AcOslow MnIII MnIII Oxidative processes fast O MnIII O -MnII Ph Ph O O MnIII O MnIII O MnII O Ph OMnIII O b) MnIII O O MnIII MnIII O MnII O Ph O Bush J. Am. Chem. Soc. 1968, 90, 5903 Heiba J. Am. Chem. Soc. 1968, 90, 5905 Ph OR cyclization Ph 60% O H Sn R3 reflux xs Br 3 Sn O O O Excluding the applications in olefin epoxidation (or alkane oxidation), Mn(III) is most commonly used for oxidative radical cyclizations. This chemistry, largely developed by Barry Snider (Chem. Rev. 1996, 96, 339) has found broad applications in the total synthesis of natural products. R AcOH + 2 MnIII MnIII Some common commercially available Mn(III) species: Mn(OAc)3•2H2O (Aldrich, $6/g) Mn(acac)3 (Aldrich, $3.1/g) MnF3 (Aldrich, $3.2/g) Mn2O3 (Aldrich, $10/g) Florina Voica O Mn(OAc)3•2H2O AcOH, 45 °C Ph O OR Heiba J. Org. Chem. 1974, 39, 3456 Applications of Mn(III) in Organic Chemistry Baran lab GM 2/6/2010 The experts: Key concepts: Barry Snider - single electron oxidant E. J. Corey - oxidative radical cyclization M. P. Bertrand - radical oxidation Janine Cossy - tandem radical cyclization Phillip Zoretic - hydrogen abstraction and others O O O H H O H 63% Paquette, Tetrahedron Lett. 1987, 43, 5567 For syntheses of upial see: Taschner, J. Am. Chem. Soc. 1985, 107, 5570 (key step: intramolecular aldol); Honda, Angew. Chem. Int. Ed. 2008, 47, 131 (key step: carbonyl ene reaction) H H O Corey, J. Am. Chem. Soc. 1984, 106, 5384 O O H O X OH Mn(OAc)3 AcOH, 70 °C X 58% X = CN (50%), only cis X = CO2Me (64%) cis:trans 4:1 O H Fristad, Tetrahedron Lett. 1985, 26, 3761 MeO2C CO2Me CHO OMOM 1. KOH, MeOH 2. Mn3O(OAc)7 (2 eq) AcOH, 70 °C (one pot) 68% yield the major isomer after a Luche reduction O H H Mechanism? R O Mn(OAc)3 AcOH CO2Me OMe O 31% Reaction mechanism O O O Wallace, J. Chem. Soc. Perkin Trans. 1, 2001, 206. OMOM H O MeO2C HO Mn(OAc)3 AcOH, 65 °C CO2Me O O O O O O Mn(OAc)3 AcOH, 80 °C CO2Me O O O O H O H CHO (±) - 14-epiupial Early paper by Corey hinted at the potential of this new methodology to efficiently assemble complex polycyclic structures. 1.3 eq Mn3O(OAc)7 AcOH, 20 min, rt Upial could not be obtained by the same strategy since the other isomer did not react in the radical cyclization! O Oxidative cyclization of β-keto acids OH Florina Voica CO2Me H Mn(OAc)3 R = H Et R = Me R Cu(OAc)2 AcOH O R CO2Me R = H 71% R = Me 56% Applications of Mn(III) in Organic Chemistry O The oxidant Mn(OAc)3 OMe fast Mn(OAc)3 - most common oxidant/initiator for these reactions. Other oxidants/initiators used: Fe(ClO4)3; CAN; Co(OAc)2 - in the termination step, its oxidative ability is limited: ∼ γ-carboxy radicals (2° and 3°) will be oxidized to carbocations ∼ tertiary radicals will be oxidized to carbocations to give alkene or to form a tertiary acetate ∼ allylic radicals will be oxidized to allylic acetates ∼ isolated 1° and 2° radicals wont be oxidized. If no oxidant is present they will be quenched by H-abstraction from the solvent or Cu(OAc)2 - oxidizes 2° radicals 350X faster than Mn(OAc)3 - reacts rapidly with radicals (∼ 106 M/sec) to form alkyl-CuIII species - 1° and 2° radicals are taken to alkenes via direct oxidative elimination from the alkyl-Cu intermediate (E-olefins and the less substituted alkene) - allylic, 3° radicals are oxidized to carbocations CuX2 (X = Cl, Br, I, SCN) - oxidize radicals to carbocations or they undergo ligand transfer Kochi, Acc. Chem. Res. 1974, 7, 351 Et Et no discrete keto-ester radical detected! OH O OMe Et solvent O O O OMe OMe Et O O O MnIIIO Me Mn(OAc)3 slow OMe O Me fast CO2Me CO2Me - MnII Et Et Et O Me Me CO2Me CO2Me H 56% cis O O O Cu(OAc)2 OMe Me Et O AcOH is the most common solvent with Mn(OAc)3. EtOH is a better H-donor than AcOH so it is preferred when vinyl radicals are involved in the termination step (vinyl radicals cannot be oxidized so they need to be quenched). O OMe The solvent DMSO, MeOH, dioxane, CH3CN can also be used but they require higher temp and the yields are sometimes lower. O O boat TS OMe slow - MnII Ac )2 O u( O Factors that determine the reaction mechanism/product distribution: MnIII O O Florina Voica C Baran lab GM 2/6/2010 or OMe Et 14% O Me O II OMe 3% H trans Cu O Me CO2Me Et H O Me CO2Me Snider, J. Org. Chem. 1988, 53, 2137 Baran lab GM 2/6/2010 Applications of Mn(III) in Organic Chemistry Radical cyclizations of β-keto esters Florina Voica Proposed reaction mechanism: a. Monocylization O R' Mn(OAc)3 (3 eq) Cu(OAc)2 (2 eq) AcOH, 80 °C O MeO OMe OMe O 76% vannusal A O Nicolaou, Chem Commun. 2002, 2480 O Mn(OAc)3 (1eq) Cu(OAc)2 (1 eq) AcOH, rt MeO2C H H O 61% H CO2Me O Me MeO O Me H O Me CO2Me OMe Me MeO2C 7% + O OH O H MeO2C triptolide O 40% Trick to improve selectivity... (we shall see more of this later) MeO OMe Me Mn(OAc)3 AcOH 70% Me CO2Me O H O H Me O O O O MeO2C H O-methylpodocarpate MeO H Me O Me Me O Me HO CO2Me 60% H R H O Me Snider, J. Org. Chem. 1985, 50, 3659 O O Zn, HCl O MeO2C Me MeO2C Mn(OAc)2•2H2O AcOH O Me R' O O H OMe Mn(OAc)3 AcOH 50% H Me b. Bicycle formation with termination onto an arene OMe O Me R' dehydropallescensin D White, Tetrahedron Lett. 1990, 31, 59 R R Mn(OAc)3 Me MeO2C Me MeO2C O Me O Me CO2Me MeO CO2Me Me Mn(OAc)3 OMe O MeO2C Me H OMe Mn(OAc)2•2H2O AcOH 90% O (±)-Triptoquinone B and C Takaishi, J. Chem. Soc., Chem. Commun. 1993, 793 Cl CO2Et Yang, J. Org. Chem. 1998, 63, 6446 Me OMe O EtO2C Cl H triptolide Applications of Mn(III) in Organic Chemistry Baran lab GM 2/6/2010 Mn(OAc)3 Cu(OAc)2 O Ph2t-BuO O O O + H H Danishefsky, J. Am. Chem. Soc. 1998, 120, 12684 N O Me OMe Cl O 60% Me N conocarpan O O Snider, J. Org. Chem. 1997, 6978 N Oxidative fragmentation-cyclization Me N mersicarpine O H Kerr, Org. Lett. 2008, 10, 1437 Oxidative cyclization of ketones O Me Mn(OAc)3 (15eq) 9:1 EtOH/AcOH 90 °C, 22h O Me + gymnomitrol NaBH4, MeOH 88% Me Me Me H Me silphiperfol-6-ene Snider, J. Org. Chem. 1994, 59, 5419 Me TMS Mn(pic)3 Bu3SnH 37% O H Me Me Me H Me HO 25% TMS Me Me Me TMS Me HO Me Me O Me O Me Mn(pic)3 DMF 58% HO Me 1. KOtBu (92%) 2. LiPPh2 (84%) OH O O Mn(OAc)3 AcOH, reflux O Cl tricycloillicinone O Mn(OAc)3 (4eq) benzene, 100 °C 25% Mechanism? O O AcOH, 50 °C 75% O O OMe O O O Florina Voica AcOH 100 °C 80% Snider, J. Org. Chem. 1997, 62, 1970 O H H Me Me DMF, 0 °C OTHP THPO H SCN Me H 10-isothiocyanatoguaia-6-ene Narasaka, Chem. Lett. 1994, 1697 Applications of Mn(III) in Organic Chemistry Baran lab GM 2/6/2010 HH H Mn(OAc)3 O 5 13 CO2Me 5 13 O O OAc H CO2Me 35% A:B/1:2 Me Me HO O Me Me 1.5% O EtO2C 2 eq Mn(OAc)3 1 eq Cu(OAc)2 AcOH 38-45% CN H O 35% yield Me CO2Et Me O Me H 3% OAc B H CO2Me Zoretic, J. Org. Chem. 1996, 61, 1806 8 H Me H CO2Me Me OAc A H CO2Me Me isospongiadiol Me H O O Me H O Me Me CN H O EtO2C Me H H H H O EtO2C H Me H Me O EtO2C Me Me O Me Me Beyer-15-ene-3,19-diol H H Me O H Me OH CO2Me H d. Tandem polycyclizations Other observed products: H Mn(OAc)3 Cu(OAc)2, AcOH O HO Mn(OAc)3 Cu(OAc)2 MeOH rt, 3h Mechanism? Me + H O MeO2C Pattenden, Synlett. 1997, 398 O EtO2C EtO2C isosteviol Snider, J. Org. Chem. 1998, 63, 7945 40% CO2Me H H Mn(OAc)3 Cu(OAc)2, AcOH 4 Me OAc O H HO H Mn(OAc)3 Cu(OAc)2, AcOH 8% CO2Me OAc Me H confirmed by X-rays Mn(OAc)3 Cu(OAc)2, AcOH 5 O H CO2Me O 37% CO2Me O EtO2C O Me H Mn(OAc)3 Cu(OAc)2, AcOH 44% Mechanism? O H H CO2Me O Me Me Me Me transanular cyclization CO2Me H H Florina Voica H Zoretic, Tetrahedron Lett. 1996, 7909 Applications of Mn(III) in Organic Chemistry Baran lab GM 2/6/2010 Me 2 eq Mn(OAc)3 1 eq Cu(OAc)2 AcOH CN 8 Me O Cl CO2Me Me Me CN H O MeO2C Cl 5% yield Me Me CF2CO2H O MeO2C Cl H H 61% (mixture of three isomers) HO Zoretic, J. Org. Chem. 1998, 63, 7213 Me O O H O HO Me Me H O 1. TBAF 2. AcCl 70% OAc H H 9-Acetoxyfukinanolide Me Me MeO Me SmI2 92% N Mn(OAc)3 Cu(OAc)2 OMe H N MeO O H O O Me MeO O H O O MeO Me 65% H O (-)-Estafiatin 5-α pregnane Mn(OAc)3 EtOH, rt O O H Zn, HCl 87% CO2Me Oxidative Radical Cyclization of 1,3-diketones H Me CO2Me O H H Me O Lee, J. Am. Chem. Soc. 1997, 119, 8391 H H Cl H α-Chloro substitution prevents overoxidation of the product! O H H Me CO2Me H Me O EtOH, reflux 65% H H Me HO O THPO O Me Me Cl CN H 70% H H H Mn(OAc)2•2H2O Cu(OAc)2•H2O THPO Me + H H H H MeO2C Cl Oxidative Radical Cyclization of 1,3-diesters CN H O OAc Florina Voica O OMe O AcOH, rt 72% OMe O OMe H O Fredericamycin A O OH H Greene, J. Am. Chem. Soc. 1996, 118, 9992 Rao, J. Chem. Soc. Perkin Trans. 1, 1993, 3171 Applications of Mn(III) in Organic Chemistry Baran lab GM 2/6/2010 Mn(OAc)3 Cu(OAc)2 O Ph2t-BuO O O O + H H Danishefsky, J. Am. Chem. Soc. 1998, 120, 12684 N O Me OMe Cl O 60% Me N conocarpan O O Snider, J. Org. Chem. 1997, 6978 N Oxidative fragmentation-cyclization Me N mersicarpine O H Kerr, Org. Lett. 2008, 10, 1437 Oxidative cyclization of ketones O Me Mn(OAc)3 (15eq) 9:1 EtOH/AcOH 90 °C, 22h O Me + gymnomitrol NaBH4, MeOH 88% Me Me Me H Me silphiperfol-6-ene Snider, J. Org. Chem. 1994, 59, 5419 Me TMS Mn(pic)3 Bu3SnH 37% O H Me Me Me H Me HO 25% TMS Me Me Me TMS Me HO Me Me O Me O Me Mn(pic)3 DMF 58% HO Me 1. KOtBu (92%) 2. LiPPh2 (84%) OH O O Mn(OAc)3 AcOH, reflux O Cl tricycloillicinone O Mn(OAc)3 (4eq) benzene, 100 °C 25% Mechanism? O O AcOH, 50 °C 75% O O OMe O O O Florina Voica AcOH 100 °C 80% Snider, J. Org. Chem. 1997, 62, 1970 O H H Me Me DMF, 0 °C OTHP THPO H SCN Me H 10-isothiocyanatoguaia-6-ene Narasaka, Chem. Lett. 1994, 1697 Baran lab GM 2/6/2010 Applications of Mn(III) in Organic Chemistry Asymmetric radical cyclization O O S 44% 100% de O Miscellaneous applications of Mn(III) salts O Mn(OAc)3 Ph Cu(OAc) 2 Me S Ph 1. oxone 2. Na/Hg O O H Me Mn(OAc)3 Cu(OAc)2 Ph O O + O MeO N O MeO O O Mn(OAc)3 AcOH 70% Ph O + O Ph (-)-virgatusin Brun, Eur. J. Org. Chem. 2009, 2306 93% O Ph Ph O Ph O O Ph Ph O O 1 eq 1.5 eq B(OH)2 O MeO B(OH)2 O MeO OMe OH O Ph Nishino, Eur. J. Org. Chem. 2008, 2404 CO2Me O MeO Mn(OAc)3 (0.1eq) AcOH, rt 85% Ph O O O 1 eq 1.5 eq O Ph MeO O NBn O Mechanism? 77% Ph Ph O MeO O Nishino, Tetrahedron Lett. 2006, 47, 7259 O O CO2Me O N Snider, J. Org. Chem. 1993, 58, 7640 O + O O Ph Mn(OAc)3 (1eq) AcOH, rt Mn(OAc)3 (3eq) AcOH, reflux Ph Ph Me N EtO Ph Nishino, Tetrahedron Lett. 1998, 39, 7931 O O Mn(OAc)3 Cu(OAc)2 28% 92% de air 1 eq tetramic acid 2eq O O Ph Ph HO Me 90% 86% de O + NBn Snider, J. Org. Chem. 1991, 56, 328 O O EtO O Ph O Florina Voica Ph Ph N O 40% yield 80% de B(OH)2 Mn(OAc)3 (3eq) benzene reflux Mn(OAc)3 (3eq) thiophene reflux Mn(OAc)3 (3eq) furan reflux 95% S 73% O 62% Demir, J. Org. Chem. 2003, 68, 578 Applications of Mn(III) in Organic Chemistry Baran lab GM 2/6/2010 Ph Mn(OAc)3 (5eq) O benzene, reflux 64% Ph O O OAc 1. NaBH4/CeCl3 OAc 2. Ac2O/Et3N O 89% Ph OAc O OH N O O Florina Voica O Mn(OAc)3 (1eq) benzene, reflux 88% O O Danishefsky, Tetrahedron Lett. 1985, 26, 3411 O O OBz OMe Br O H OEt Mn(OAc)3 benzene reflux, 24h 67% Mn(OAc)3 (1eq) benzene, reflux OBz AcO OMe Br 91% N OH O H O Demir, Tetrahedron Lett. 1997, 38, 7267 OEt Watt, Synth. Commun. 1989, 19, 1127 CHO For a review on methods of α'-oxidation of enones see: Demir, Synthesis 1991, 235 OH OH MeO N H Me CN NMe H OMe N O H MeO Me H H O OMe EtO2C AcOH CO2Et MeO CO2Et 4 eq MeO O H H O CO2Et N t-BuHN CO2Et O O N t-BuHN MeO MeO MeO MeO 59% (±)-Cyanocycline A OTBS O TBSO H OMe CO2Et N H Proposed reaction mechanism: NMe O Mn(OAc)3 (0.1eq) TBHP (5eq) CN H N O H + NH2 N H 55% OTBS O H OH O Mn(OAc)3 (xs) 0.3% H2SO4-ACN rt, 2h Fukuyama, J. Am. Chem. Soc. 1987, 109, 1587 TBSO NC O 1. MeOH, rt 2. Mn(OAc)3 (4.5eq) AcOH OMe O O t-BuHN H 72% H H H O O O N 1,4 aryl transfer MeO OMe Shing, Org. Lett. 2006, 8, 3149 MeO EtO2C CO2Et CO2Et EtO2C t-BuHN O N MeO MeO EtO2C CO2Et Baran lab GM 2/6/2010 O Applications of Mn(III) in Organic Chemistry O O N t-BuHN Mn(III) AcOH MeO MeO t-BuHN CO2Et EtO2C O OH OAc N MeO H2O O TMS CO2Et OMe CO Et 2 CO2Et CO2Et MeO Mn(III) O OMe CO2Et Vieu, Org. Lett. 2007, 9, 4171 Mn(OAc)3•2H2O (0.1eq) AcOH (2 eq), MeOH, 40 °C MeO Ph 90% Mechanism? 1.5 eq H N Me CO2Me Narasaka, Org. Lett. 2008, 10, 5019 OH Ph O CO2Me O CO2Et 5-exo-trig indane O N CO2Me CO2Me Mikami, Synlett. 2002, 1868 NH MeO MeO OH MnF3 (1.2eq) DCM 61% O N H N3 + via O + CO2Me Florina Voica 1.2 eq (slow addition) Mn(acac)3 (0.1eq) MeOH, rt, 1h Mechanism? HO Ph (1.5 eq) Ph N3 Mn(acac)3 (1.7eq) Ph MeOH, rt, 5 min then AcOH (2eq), rt, 1h N Ph 84% Chiba, J. Am. Chem. Soc. 2009, 131, 12570 Conclusions: - oxidative tandem cyclization provides access to complex carbon skeletons - stereo- and regiospecific method for rapid assembly of polycyclic structures from simple linear precursors - numerous applications in natural product synthesis - Mn(OAc)3 selective, mild oxidant - very little chemistry of Mn(III) salts other than Mn(OAc)3 Underdevelopped aspects of this chemistry: - efficient asymmetric radical cyclization - catalytic oxidative radical cyclization - model studies to understand the tandem cyclizations better - more creative examples for the termination step