Year in Review: Journal of Organic Chemistry 1986
advertisement
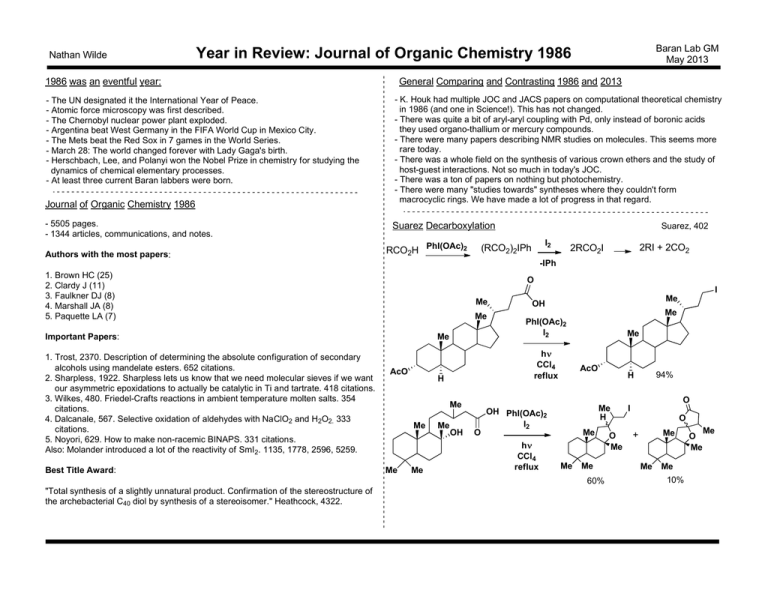
Nathan Wilde Baran Lab GM May 2013 Year in Review: Journal of Organic Chemistry 1986 1986 was an eventful year: - The UN designated it the International Year of Peace. - Atomic force microscopy was first described. - The Chernobyl nuclear power plant exploded. - Argentina beat West Germany in the FIFA World Cup in Mexico City. - The Mets beat the Red Sox in 7 games in the World Series. - March 28: The world changed forever with Lady Gaga's birth. - Herschbach, Lee, and Polanyi won the Nobel Prize in chemistry for studying the dynamics of chemical elementary processes. - At least three current Baran labbers were born. Journal of Organic Chemistry 1986 - 5505 pages. - 1344 articles, communications, and notes. Authors with the most papers: General Comparing and Contrasting 1986 and 2013 - K. Houk had multiple JOC and JACS papers on computational theoretical chemistry in 1986 (and one in Science!). This has not changed. - There was quite a bit of aryl-aryl coupling with Pd, only instead of boronic acids they used organo-thallium or mercury compounds. - There were many papers describing NMR studies on molecules. This seems more rare today. - There was a whole field on the synthesis of various crown ethers and the study of host-guest interactions. Not so much in today's JOC. - There was a ton of papers on nothing but photochemistry. - There were many "studies towards" syntheses where they couldn't form macrocyclic rings. We have made a lot of progress in that regard. Suarez Decarboxylation RCO2H PhI(OAc)2 Suarez, 402 I2 (RCO2)2IPh 2RI + 2CO2 2RCO2I -IPh 1. Brown HC (25) 2. Clardy J (11) 3. Faulkner DJ (8) 4. Marshall JA (8) 5. Paquette LA (7) O I Me Me Important Papers: 1. Trost, 2370. Description of determining the absolute configuration of secondary alcohols using mandelate esters. 652 citations. 2. Sharpless, 1922. Sharpless lets us know that we need molecular sieves if we want our asymmetric epoxidations to actually be catalytic in Ti and tartrate. 418 citations. 3. Wilkes, 480. Friedel-Crafts reactions in ambient temperature molten salts. 354 citations. 4. Dalcanale, 567. Selective oxidation of aldehydes with NaClO2 and H2O2. 333 citations. 5. Noyori, 629. How to make non-racemic BINAPS. 331 citations. Also: Molander introduced a lot of the reactivity of SmI2. 1135, 1778, 2596, 5259. Best Title Award: AcO Me Me OH Me Me PhI(OAc)2 I2 H h CCl4 reflux Me OH Me AcO O Me H OH PhI(OAc)2 I2 h CCl4 reflux Me Me Me 60% "Total synthesis of a slightly unnatural product. Confirmation of the stereostructure of the archebacterial C40 diol by synthesis of a stereoisomer." Heathcock, 4322. 94% H O Me Me Me O Me I O Me + Me Me 10% O Me Me Baran Lab GM May 2013 Year in Review: Journal of Organic Chemistry 1986 Nathan Wilde Synthesis of Compactin Keck, 2487 HO THPO OMe Me HO 1. LAH 2. I2 3 steps H Me C Me C O N O Cyclooctenes k cis/k trans Me "Rebek peracid" Me Me tBu "vinylallene" 75% Me O O Me PPh3 Me H O OEt O N O Me O nBuLi, TsCl; OLi PPh3 O C Me HO Rebek, 1649 Selective Peracid Epoxidation tBu tBu Et Et k cis/k trans Et k cyclic/k acyclic k endo/k exo Me OAc OH OAc h 2. TBSCl 3. O3 9 steps O MeO O OAc O OTBS SnBu3 1. MeO O TBSO 2.2 mCPBA 1.2 1.0 0.84 1.1 Rigby, 3298 4 steps AcO OMe H OH H 84% C 55% 36% AcO O R = OMEM Me OMe Br LDA OMe R 1. Me2CuLi; H3O+ O 2. NaH, toluene reflux O 66% HO Me O H O Me H R 48% Me O O O Me O Me 2 Me 1. 2. aq. HCl 3. Ag2CO3, celite R O O 1. 2. LiB(sBu)3H H 1:1 mix of diastereomers 3.2 O O "vinylallene" 74% 7.8 O OMe O 7.7 Towards Ingenol? OPh S TBSO "Rebek peracid" Me (+)-compactin H 1. TMSCl, NaI 2. PDC 56% O Me O O H H Me Me HO Me Me HO HO ingenol OH Convergent Vitamin A Synthesis Me Me Otera, 3834 SO2Ph 1. nBuLi, then Me OAc Me 2. EtOCHCH2, H Me Me Me Me O Me Boger, 3250 N N Me E MeO2C E 9 steps CO2Me MeO N N OEE 100°C, 71% MeO Me O TBSO Me OTBS N N OH 38°C MeO N N OAc + Diels-Alder Reactions of Heterocyclic Azadienes Me SO2Ph Me O Me MeOK cyclohexane Baran Lab GM May 2013 Year in Review: Journal of Organic Chemistry 1986 Nathan Wilde 230°C, NAc NAc 83% yield 95:5 all trans : any cis 82% Me MeO vitamin A MeO O 1. AcOH 2. MnO2 3. NaOMe O N3 O O OMe MeO O Baeyer-Villiger Alternative OH Me cat. TsOH 10 eq. H2O2 H O O xylene N3 Boger, 5436 NAc Me MeO OH reflux 56% HN NAc 1. HCl, MeOH 2. NaBH4 3. H2O2, Ac2O Me MeO O O Me H2N OMe mCPBA HN OAc OH Me OMe OMe NAc MeO OMe OH CC-1065 N PDE-II 1. cat. TsOH 10 eq. H2O2 O OH OBn Me 2. BnBr 86% (2 steps) OH NH 60% OMe O N O O OMe Me NH N OMe O N H O OMe Minisci Reaction: Polar Effects N CO2Me O N 60°C Bn 51:49 N Bn N CO2Me OHC NH CO2Me 1. AgBF4 then LiBr 2. DBU CN N O OAc MeO2C H H O H N MeO2C O CO2Me OSEM O MeO2C 1. LiBHEt3 2. TBSCl TBSO 3. mCPBA MeO O MeO O MeO2C OAc O MeO O OSEM Me 5 steps PhS O O H OSEM Me O lycorine N O MeO O O H O OSEM Me OMOM KHMDS TESCl O HO2C Me O MeO O O H O O OSEM Me OMOM 1. Cl2POOPh PhSeH 2. Bu3SnH AIBN O MeO O O H O Me Me OMOM AgTFA Na2HPO4 O O HO MeO O O H Me Me N O HO TBSO O O MeO OSEM Me "acid chloride" DMAP Me 1. LiMe2Cu 2. SEMCl TBSO 3. H2, Pd/C O BnO OAc O LiHMDS then OBn MeOSO F 2 O OH HO H O 1. butadiene 2. MeOH 3. CH2N2 OMOM O O O mechanism? OAc O O BnO HClO4 then KCN O CO2Me O O Boeckman, 3740 O OBn O 18:82 1. K phthalimide 2. Fe(CO)5 3. OsO4, NaIO4 4. MeO2CCH2CO2H 5. NH2NH2-nH2O O "acid chloride" H O Towards Lycorine Cl Me 7 Explanation: Subsequent H abstraction is faster in H2O. This might be because of HSAB theory applying to radicals. tBu O O O perkadox 16 O O O tBu O 20 steps Me toluene perkadox 16 MeCN/H2O (1:1) 60°C "Epimerization of the C-7 center was delayed ... For the sake of convergency, we felt that inversion of this center be completed as early as possible." COCl H H N Bn O Ireland, 635 Towards Chlorotricholide Minisci, 476 Bn toluene perkadox 16 N Baran Lab GM May 2013 Year in Review: Journal of Organic Chemistry 1986 Nathan Wilde OMOM OMOM Formal Synthesis of Asperdiol Marshall, 858 H O 9 steps O Baran Lab GM May 2013 Year in Review: Journal of Organic Chemistry 1986 Nathan Wilde O O Me Me BnO SO2Ph "sulfone" I Me 1. p-benzoquinone 2. h 5 steps OTBS Me "sulfone" H Me OTBS H O H HO not this H Me Me Me BnO SO2Ph 1. DIBAL 2. BnBr 3. TBAF 4. I2, PPh3 Me Me BnO OTBS SO2Ph 1. KHMDS 18-c-6 2. Na, NH3 3. PhCOCl H I Me Me OBz OH Pyridone Synthesis H Ph O Me HO Me Ogliaruso, 1544 Ph Ph Ph Me HO Me Me Ph H+ NaN3 Ph Ph N N NH Me tBuOK MeI Ph Me O Me asperdiol + H Mechanism Taylor, 101 O H3O+ N O H OBn CO2Me 25% H FVP 25% O NaBH4 O 700°C 46% OTBS Me 69% CO2Et 88% O CO2Et H H2, Pd/C CO2Et PPh3 O (EtO)2P O O CO2Me 1. PPh3 2. KHMDS ClCO2Me Marchand, 1897 Synthesis of 1,3-Bishomopentaprismane OAc NO N N O N N Discovered during functionalizations of 2-nitrosopyridine N Ph Ph NMe Me Ph OH Me O Ph N N NMe Ph O Ph Ph N NMe Me O Milbemycin Synthesis N Me Barrett, 4840 Me nBuLi (3 eq) O NHTs H N Me OH H N Cy Me O SO2Ph 7 steps Cy Me O (+)-citronellene Me N other isomers O [3,3] N O Me Me Me Me 73% resolution and separation of diastereomers. They commented: "While on an analytic scale resolution was straightforward, preparative HPLC was at best synthetic lunacy." N O Me 82 Me 14 4 15 81 4 Me Me [3,3] O O O C(OMe)3 Me OTBS Me Me Me Me They had another route that involved a O Kurth, 1377 Me "sulfone" Me 4 steps Me Aza-Claisen Utility Me O Me O Baran Lab GM May 2013 Year in Review: Journal of Organic Chemistry 1986 Nathan Wilde O O MeO N H O 75% 8 steps O O Me Me + then H 84% O nBuLi "sulfone" PhO2S Me Me O O O 3 steps CO2Et CO2Et O S 47% CO2Et OAc S CO2Et Me OTBDPS OTBS O Me Me OMe nBuLi O Conjugate Reduction O Me Me then TFA, TBAF MacDowell, 183 250°C E:Z = 5:3 Me OTBDPS CO2H Mechanism OTBDPS Me O OTBDPS O O Me O O O O O Me OMe (+)-milbemycin O Me "MeCu" DIBAL H H H 1. KH, 18-c-6 Me 2. DEAD, PPh3 3. NaSEt H THF/HMPA 5:1 -50°C H O H HO Me Me H 84% Me Me H Me Saegusa, 537 + H H H H H O 6% "MeCu" is premixed MeLi with CuI. No mechanistic explanation is offered. Me 3 OH Me Tufariello, 3556 Lolium Alkaloid Synthesis "These alkaloids have not yielded to the thrusts of several synthetic attempts, although the skeleton has been assembled." OMe O CO2Me HO CO2Me MeO HO OAc OTBS TBSOTf PPh3 CO2Me nBuLi iPrCHO N OAc OH N 1. Jones 2. H+, EtOH 3. NH2NH2 4. RONO 5. LAH OTBS O TBAF Me Me PPh3 Me OMe CO2Me OH NaOMe O N O O OMe CO2Me MeO OH 1. MsCl 2. H2, Pd/C N O Cl Kozikowski, 3400 Phosphonosilylation/Wittig N N N MeO Baran Lab GM May 2013 Year in Review: Journal of Organic Chemistry 1986 Nathan Wilde O 6 steps 1. TBSOTf, PPh3 2. nBuLi H nPrCHO Me TBSO OTBS CO2Me DMAD 81% NHMe CO2Me 79% overall N 98% nPr nPr O HCl CO2Me loline CO2Me nPr Chiral Directed Lithiation N Gawley, 3076 tBuLi O N N Li N N Me 90% yield 90% de Me MeO N O 1. tBuLi 2. MeI N Me Me O PPh3 N Me NH2NH2 O LDA R'CHO 100% PPh3 RCHO R' R Me 93% Me EtO P EtO R R = R' = Ph : 70% yield R = R' = nPr : 83% yield R = nPr, R' = Ph : 20% yield + other products Me N Ph MeO MeO O EtO P EtO LiP(O)(OEt)2 Me MeO MeO Minami, 3572 O Me N Me Me MeO MeI O One-Pot Diolefination Ph DMAD MeO2C CO2Me MeO2C CO2Me NH (+)-salsodine Me Ph Ph Deoxygenation Leone-Bay, 2378 O Me OH Ph2PLi Me Me Me Ph2P Halide Reduction Me OH MeI OH Me R-X Ph Me DMBI+ X- O 72% O EtO2C R CO2Et N 50°C R N3 R silica R N N Rh2(OAc)4 93% "Furthermore, the possibilities of dihydropyridines as practical reducing agents in organic synthesis seem to be somewhat low because of their poor reactivity and their instability is a disadvantage to their use." -Oxidation of Iminium Groups CO2Et R O NH O O Me Polymer-Supported LAH Reduction H Frechet, 3462 tBuNHOH Me Me 1-x x N Cl HO OH O Me -15°C Me Me ArO ArO H Al Me N O Li Ph Ph DF = 28-35% 33% ee 84% yield tBu O R H R O Cl Me DF = 9% 78.8% ee 97% yield Me N tBu H Me Ph LAH ArOH l-ephedrine N Me 1-x x Ph Ph Coates, 1383 CO2Et R R 1-x 80% NH N x H Cl R R R N N DMBI H This reagent was originally used to isomerize olefins through their respective epoxides. Cis-cyclooctene to tr ans-cyclooctene. Fuchs, JOC 1973, 1178. Sha, 1120 EtO2C O O Br DMBI 1,2-dehyrdoisoquinoline Synthesis + R-H Me DMBI all one-pot X N Me Me O N N O Me OH Me N Ph OH Chikashita, 5400 Me Me MePh2P OH MePh2P Baran Lab GM May 2013 Year in Review: Journal of Organic Chemistry 1986 Nathan Wilde R O varied functionalization R O N O tBu O [3,3] H Me tBu H Me Me Me N Me 23 citations. Sorensen used it in a synthesis of fumagillol; he called this an "underutilized method." ACIE 1999, 971. Year in Review: Journal of Organic Chemistry 1986 Nathan Wilde Towards Ikarugamycin O H H 1. LDA, TMSCl 2. O3, then DMS 3. MeOH, H+ OMe H H O ArSO2Cl, then DMAP, O H3N OMe NHAlloc MeO H Me O Me O H O MeO2C H NHAlloc H O 1. H+ 2. tBuOK, Me O O (EtO)2P OMe MeO2C NHAlloc H H O OK Me O O N H Me O 1. Pd(PPh3) 2. H2, Pd-BaSO4, quinoline 3. ArCHO, NaBH3CN H Me O O H MeO2C N Ar H HN PhMe, H O N H O OH O O H H MeO2C H 9 steps H H O Boeckman, 5486 MeO H OMe O HN O N Ar KOtBu O H H N Ar H OMe HN OH O O Me HH H Et H H O H N OMe H HN O ikarugamycin Baran Lab GM May 2013