JACS Klement Foo Baran GM 1989
advertisement
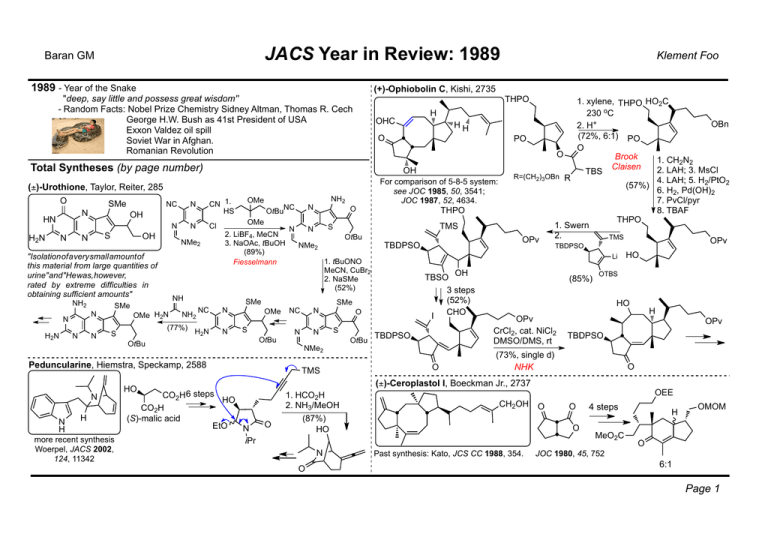
JACS Year in Review: 1989 Baran GM 1989 - Year of the Snake (+)-Ophiobolin C, Kishi, 2735 "deep, say little and possess great wisdom" - Random Facts: Nobel Prize Chemistry Sidney Altman, Thomas R. Cech George H.W. Bush as 41st President of USA Exxon Valdez oil spill Soviet War in Afghan. Romanian Revolution THPO N H2N N S N OH N N H2N N N OMe H2N (77%) OtBuNC N N N 2. LiBF4, MeCN 3. NaOAc, tBuOH (89%) Fiesselmann NC N H2N N NH2 OMe OMe NH SMe S Cl NMe2 "Isolationofaverysmallamountof this material from large quantities of urine"and"Hewas,however, rated by extreme difficulties in obtaining sufficient amounts" NH2 N OtBu N N H H more recent synthesis Woerpel, JACS 2002, 124, 11342 NH2 OMe S OtBu O For comparison of 5-8-5 system: see JOC 1985, 50, 3541; JOC 1987, 52, 4634. THPO TMS S OtBu NMe2 TBDPSO NC N N N O NMe2 TMS OtBu TBDPSO OH TBSO 3 steps (52%) CHO I SMe S 1. xylene, THPO HO2C 230 oC OBn 2. H+ (72%, 6:1) PO PO O O Brook 1. CH2N2 Claisen 2. LAH; 3. MsCl TBS R=(CH2)3OBn R 4. LAH; 5. H2/PtO2 (57%) 6. H2, Pd(OH)2 7. PvCl/pyr 8. TBAF THPO 1. Swern 2. TMS OPv OPv Li 1. tBuONO MeCN, CuBr2 2. NaSMe (52%) SMe Peduncularine, Hiemstra, Speckamp, 2588 HO HH O OH CN 1. HS N H OHC Total Syntheses (by page number) (±)-Urothione, Taylor, Reiter, 285 O SMe NC N OH HN Klement Foo TBDPSO O (85%) HO OTBS HO OPv CrCl2, cat. NiCl2 DMSO/DMS, rt H OPv TBDPSO (73%, single d) NHK O (±)-Ceroplastol I, Boeckman Jr., 2737 CO2H 6 steps CO2H (S)-malic acid HO EtO O N iPr 1. HCO2H 2. NH3/MeOH (87%) HO N O CH2OH OEE O O O Past synthesis: Kato, JCS CC 1988, 354. 4 steps MeO2C H OMOM O JOC 1980, 45, 752 6:1 Page 1 JACS Year in Review: 1989 Baran GM OEE MeO2C O O H H OMOM MeO2C OMOM Na, NH3/THF tBuOH; NaBrO3 (NH4)2Ce(NO3)6 cat. then CrO3 acetone; CH2N2 excess (45%) CO2Me KN(TMS)3 E then LiI, collidine, Δ (76%) Dieckman O H O (–)-Picrotoxin, Yoshikoshi, 3728 CHO O O OO OH NBS OHC O (89%) MeO2C NaOMe MeOH (73%) O H O OH 2 steps OMe Klement Foo 1. MeLi 2. Ac2O, pyr DMAP (91%) Br Br Other synthesis: Corey, JACS 1979, 101, 5841 O O AcO H OMs O Na[PhSeB(OEt3)] EtOH (92%) AcO NBS (88%) O 1. Zn(Cu) NH4Cl (88%) OAc 2. Jones O O TL 1987, 28, 4293 OH O OAc OAc Br (±)-Oxosilphiperfol-6-ene, Kakiuchi, 3707 AlCl3 (93%) O OTMS Other syntheses: Paquette, JACS 1984, 106, 6690; Snider, JOC 1994, 59, 5419; Rawal, OL 2000, 2, 2711. 3 Steps (63%) MeO AcO O O 1. H2O2, NaOH/MeOH 2. N2H4.H2O, AcOH 3. PCC (51% overall) Wharton a O O O 4 steps (32%) O 4 steps (76%) O Cargill O O in this paper O C4H9 (±)-silphiperfol-6-ene O Os2O4 OH O pyr then O AcO H2S (86%) O Br O 3 steps Br (–)-Perhydrohistrionicotoxin, Winkler, 4852 C5H11 C5H11 O HN HN 6 steps HN O OH b 6 steps O OH 3 steps OH O O 1. NaBH4 2. NaH (60%) C5H11 hv HN O O (95%) Br Other syntheses: Keck, JOC 1982, 47, 3590; Corey, JACS 1975, 97, 430; Evans, JACS 1982, 104, 3695 (–)-picrotoxin O OO O O (–)-picrotin C5H11 HN O O O O O O Page 2 JACS Year in Review: 1989 Baran GM Pseudopterosins A & E, Corey, 5472 1. DIBAL-H 2. Ph3P=C(Me)SEt 3. Swern 4. HgCl2 5. NaOMe, MeOHO (40%) H H O O OSugar H H KH-HMPA TBSCl (97%) 1. 2-butynal TMSOTf 2. PCC (61%) TBSO KH O (70%) O 1. (PhSe)2O, (Me3Si)2NH 2. HClO4, H+ (56%) H H O OH OH (±)-Dactylol, Feldman, 6457 K 1. O OH PhO2S [6π+2π] O hv (41%) 2. LiCH2SO2Ph (31%) OH H2, Pd/C OH HO LAH (73%) (89%) H OMe OH H isolable OH O O OH OH NHAr OMe OTBS 1. MeI K CO 2 3 O O 2. H+ (63%) OH Hoffmann OH O NHAr O H H (50%) O 11:1 PhC(Me2)O2Li 1. 3.6N HCl CO2Me 2. Bu3P, HO DEAD (28-31%) H H O OH OH Pd2(dba)3 (allyl-O)2CO Δ, 55% ∗ H O OH E H O O H H 15% of cis-syn-cis 14% of regioisomer Study towards: Kinsella, JOC 1990, 55, 105. O O H Salcomine, O2, MeCN (72%) OH OMe CHO OH MeOH CaCO3 NO23 d, 95% Bradsher cycloaddition Cl BCl3 (77%) (–)-Echinosporin, Smith III, 8039 H O O O HO O CONH2 O O H O H H O OH O2N O O O OH O H O H O OMe O TL 1988, 29, 3423 H N OH O OH O HO (–)-Cryptosporin, Gupta, Franck, 7668 OH O OMe Me O H OH Klement Foo HO E H CONH2 SO3, pyr DMSO O (46%) OH H HO E H H NH4OH, OH MeOH CONH2 (86%) O O H OH OH via fragmented diketone Page 3 JACS Year in Review: 1989 Baran GM (–)-Aspochalasin B, Trost, 8281 OH E E 17 steps for required oxidation O level and 3 more side chain Cs. BHT,Δ O N H N H NHCbz (33-40%) H N H OH O O O O O (Unexpected) Outcomes in Methodology Sworin, 1815 O OH H OH O N H RCM to the rescue!!! Methodology: Invention and Study 2 steps 1. (EtO)2C=CHCO2Et, PPTS, OCO2Me 2. KOH; reflux; CH2N2, O OEt 3. BuLi-DIBAL-Hl; PCC SO Ph 2 4. [CH2=C(OEt)]CuLi; O ClCO2Me; (10%) O SO2Ph OH Cl O [3,3] Δ Cl Paquette, Schreiber, 2331 H N H via: H Pd(PPh3)4 via syn π-allyl dppp, reflux Pd complex (49%) CH3CO3H, K2CO3; PPTS O OEt (62%) O SO2Ph Klement Foo BnNMe3F O N H O O SO2Ph OH (53%) H OH KHMDS 4 d (50%) NP OH OMe O KH (40%) O via: H O H OMe ThD single diastereomer (±)-Pleuromutilin, Boeckman Jr., 8284 OTs OH 1.Me2CuLi, HMPA 2. (CH2=CH)MgBr (80%) O O O O OH MeO Hayashi, Ito, 3426 OH KH Δ (99%) Anionic Oxy-Cope O HB Ar H2O2 O cat. [Rh(cod)2]BF4 NaOH (+)-BINAP Ar OH ee 82-96% except at RT 57-76% -complements normal hydroboration regioselectivity -only applicable to Rh cat. with 3o phosphine L and net + charge on Rh Page 4 JACS Year in Review: 1989 Baran GM Trost, 4430 via: OPd+ Pd2(dba)3.CHCl3 PPh3, O AcO Moore, 975 O R1 O TMS Δ R2 O O O O more hindered Rozen, 8325 F2, MeCN 0 oC H Ph R R= alkyl, Ph aldehydes E/Z-olefins (at will) ketones 1. BMS R O 2. MeOH Ph 3. H2O2/OH (50%) N H Ph R 1. 9-BBN 2. MeOH Ph Me MeOH RH2N Ph H B Me OMe O O O R OEt R O E R2 O R Bn R H O Me O Me OH O O O MeO Padwa, 6451 H O Rh2OAc4 N N E E O R N2 O R If an R group replaces H, the 5,5,5 system does not form Few variations of alkyne O O O Isoarnebifuranone Me R O Bn O cis H elim. B(OR)2 E R1 O E R O R2 O O Bn R Bn R R O R1 O R1 E R MeO R (81%) trans elim. OE R2 R R R2 Selectivity between 5 or 6membered ring is dependent on R. Radical stabilizing groups favor cyclopentadione. Electrophile E affects outcome =>bicycle can form In the next paper: Ph E selectivity: O R2N R N H H H O 2 rotate Ph H B(OR)2 2 2 H B(OR)2 R2N H Ph Me Ph Me Z selectivity: R2N H H B R1 R R2 OE Example OH (Interesting) Selectivity Issues in Methodology O R1 O O O H H (80%) Brown, 384 R Klement Foo O nanaomycin D O O O E (<10%) N E R (90%) H O E N N E H+ O R E E R O E E N R Page 5 O JACS Year in Review: 1989 Baran GM R1 R2 R3 ZnBr Yamamoto, 366: Inverse polarity Kanematsu, 5312 O Klement Foo tBuOK tBuOH Δ 0.5-1 h R α O [4+2] R1 2 R3 O R1 O ZnBr [2+2] R2 R3 R2 R3 [3,3] R1 R O S-trans "Counter-intuitive"-chemistry in Methodology: R1 O TMS O O OMe Anti-Bredt lactams possible due to amide tBuO N ability to assume Td geometry Rh2OAc4 H N (50%) H O R O OTMS Cl Other Methodology: Bailey, 765 tBuLi I -78 oC Li Li NH MeOH I 2 steps -unprecedented in 1989 -deoxygenated solvents needed O Schwartz, 3069 RO2C HO NH N2 O R1 CO2H R2 O O=VCl3 O -2HCl R1 -Limited substrates -Stoichiometric in V V 73-81% 60% for CO2 Cl O R2 Li TMEDA to RT Li E+ O O TMSCl O Williams, 1073: "Anti-Bredt lactam" 1. Na/NH3 OH O PPh3. 2. O3/Me2S HO2C DEAD then Jones NHOMe (85%) (64%) N O -Few examples of Nu: other alternatives are geranyl zinc bromide and benzyl zinc bromide -Electrophile is limited to D2O, I2, MeI, alkyliodides TMSCl as E+: O R3 O E+ R O ZnBr O R2 O Ni cat. R R2 R3 S-cis Depends on R1 R1 O β E Δ -CO2 O Examples R2 R1 R1 R2 Ph 73-95% Page 6 JACS Year in Review: 1989 Baran GM Brown, 1754 1. NaOtBu ∗ R 2. BrCH2CO2Et 1. NaOtBu 2. ClCH2CN ∗ R BR2 (9-BBN) ∗ R ∗ 1. NaOtBu R 2. BrCH2C(O)R' Examples of R: CN Et CO2Me CO2Me 2. CH2N2 (81%) -ortho-lithiation not suitable with methyl ester -double ortho-lithiation not common -not limited to aromatic systems CONiPr Et CONEt2 1. CO2, Δ E xs. (TMP)2Mg 2. CH2N2 CONEt2 E CONEt2 E CONEt2 (87%) -Buchwald Zr chemistry: Pages 776 and 2870 (see Maimone GM) -Pd cat. cross-coupling: Negishi, 3089; 3336; 3454; 8018 -SmI2 promoted reductive couping: Molander, 8236 -TFDO oxidation: Curci, 6749 -Diels-Alder related: eg. Corey 5493 -Sharpless Dihydroxylation related -Trost Pd-TMM related: eg. 7487. -Nicolaou Brevetoxin B related: eg. 5321, 5330, 5335... -Stryker's reagent-conjugate reduction, 8818. -Aldol related CONiPr N -possible on cubanes E Pearson, 6778 cf. R H R RO Fe(CO)3 O Nu R Nu R Nu [InMo(CO)3]2 AgBF4 1. Li/NH3 2. conc HCl Δ 1:3 -nucleophile has to be soft. GR just deprotonates [Mo] O H R N H H OH O OMe H E O OH (±)-trichodermol, Pearson, 1499 HO H N N O PF6- then alkylation Total Syntheses (not covered) CONiPr Fe(CO)3 O NOPF6 NEt3 Methodologies (not covered) CONiPr RO O -Nucleophiles can be lithium enolates or Grignard reagents -dienes in 7-membered rings shown -symmetrical dienes shown only -high yields for nucleophlic attack Eaton, 8016 1. CO2 xs. (TMP)2Mg CO2H air KOH Nu H R' H [Mo] CO2Me O 99% ee CO2Me [Mo] [Mo] CO2Et Klement Foo E triquinocene, Cook, 2169 E N N H H (+)-koumine, Magnus, 786 PhO2S 3 H O N N Pr Pr H E (±)-pumiliotoxin C, OMe Balasubramanian, 3363 Page 7 JACS Year in Review: 1989 Baran GM Klement Foo Total Syntheses (not covered) cont'd OH HO NH H O NH2 OH OH N N NH Huperzine A, Kozikowski, 4116 Et OH O Pancratistatin, Danishefsky, 4829 O O Vallesamidine, Heathcock, 1528 O N H O O N NH N N H O (+)-croomine, Williams, 1923 Daphnilactone A, Heathcock, 1530 (±)-Suaveoline, Cook, 7504 and many more... Interesting Structures Large molecular bowl, Pascal Jr., 6846 Cyclophane hosts, Whitlock Jr., 692 [4]Radialene system, Iyoda, 3761 Page 8