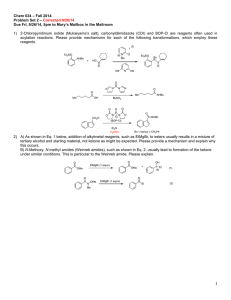
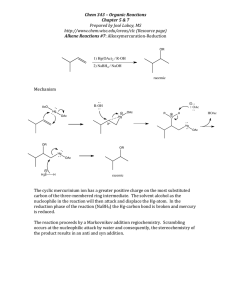
The Chemistry of Barry B. Snider Barry B. Snider Paul Krawczuk
advertisement

Baran Group Meeting The Chemistry of Barry B. Snider Born: -January 13th, 1950, Chicago, Illinois Education: -B.Sc. Chem. University of Michigan, Ann Arbor, MI, 1970 -Ph. D.: Harvard University, Cambridge, MA, 1973 (advisor: E.J. Corey) -PostDoc: Columbia University, New York, NY 1973-1975 (advisor: Ronald Breslow) Paul Krawczuk Barry B. Snider Independent Career: -Assistant Professor, Princeton University, 1975-1981 -Associate Professor, Brandeis University, 1981-1985 -Professor, Brandeis University, 1985-present Research Interests: -Total synthesis of biologically active natural products with structural novelty of the ring system or functionality -Method development especially oxidative free-radical cyclizations, ene reactions, Lewis acid catalyzed reactions 1 The Chemistry of Barry B. Snider Baran Group Meeting Paul Krawczuk Allylic Cyanobis(methylthio)methylation. Insertion of a Functionalized Carbon in an Allylic Carbon-Hydrogen Bond. JACS 1976, 98, 7116-7117. S S MeS SMe S CN CN 110°C, PhMe sealed tube BuLi or LDA MeI CN 3:1 THF:HMPA SMe SMe CN SMe CN Raney-Ni EtOH 72% 83% 83% Nickel-Catalyzed Addition of Grignard Reagents to Silylacetylenes. Synthesis of Tetrasubstituted Alkenes. JACS 1978, 100, 4624-4626. R TMS Me MeMgBr 10% 1:1 Ni(acac)2/AlMe3 R M TMS Me E+ 48-75% R E TMS Me R TMS various electrophiles used: Br E HCHO I2 TMS group allows for separation on silica, without it the olefin isomers are inseparable Reactions of Phenylhydrazones with Electron-Deficient Alkenes. JOC 1979, 44, 218-221. Ph NPh Ph N N hydroquinone N H CN in contrast to: NH 120°C, PhH N CN 71% CHO Ph MeI 30°C, PhH with vinyl bromide a diene is formed, presumably through a nickel catalyzed process. CO2 Br Ph HN N O2N also with methyl acrylate in 48% Ph N N air Ph Ph NO2 NO2 Lewis Acid Induced Conjugate Additon of Alkenes to !,"-Unsaturated Ketones and Aldehydes. JACS 1980, 102, 5872-5880. O 2 equiv. EtAlCl2 O OAlH H deuterium labeling studies show that there are two 1,2-hydride shifts EtAlCl2 also O -AlO H H O 2 The Chemistry of Barry B. Snider Baran Group Meeting Paul Krawczuk C-20 Stereospecific Indroduction of a Steroid Side Chain. JACS, 1981, 103, 1293-1295. O CO2Me 1) EtPPh3I, KOt-Bu CO2Me EtAlCl2 2) Ac2O, Pyr AcO HO AcO 2) Ac2O, Pyr 80% HO HO 1) HCHO EtAlCl2 O steps OAc O 8:1 trans:cis OH AcO Pseudomonic Acid A (formal synthesis) Total Synthesis of (±) and (-)-Ptilocaulin. JACS, 1984, 106, 1443-1445. O 1) CuBr-DMS O O Br R LDA Me BrMg HN O NH2 O O O nBu HCl, Dioxane O 2) H2, Pt/C COOH 8 O O OH O CO2Me AcO 89% Total Synthesis of (±)-Pseudomonic Acids A and C. JACS, 1982, 104, 1113-1114. 1) HCHO Me2AlCl H H2, Pt/C EtOAc nBu HN Me Me HN NH NH2 nBu PhH reflux Me Synthesis of the Hexahydronapthalene Moiety of (±)-Compactin. JOC, 1982, 47, 2682-2684. Br HO MeMgBr 6 mol % CuCl OH R H HO O Me L-Selectride 75% 3 steps (formal synthesis) 2) BrMg 80% H NaOMe LiAlH4 R R 1) Swern R OH R Me PhH 150°C sealed tube • R O OH 60% PCC-NaOAc 40°C KOt-Bu/HOt-Bu • R OH 50% 3 The Chemistry of Barry B. Snider Baran Group Meeting An Approach to the Synthesis of Neplanocin A. JOC, 1985, 50, 1983-1985. Br Br O O O OsO4 DBN O Br Br Paul Krawczuk could not be elaborated N 2 100% BnN 70% BnN BnN 62% N BnN Br Neoplanocin A HO OH OH HO OH Manganese(III)-Based Oxidative Free-Radical Cyclization. Synthesis of (±)-Podocarpic Acid. JOC, 1985, 3659-3661. OMe OMe OMe O Me Mn(OAc)3 HOAc 70% O Me Me Zn, HCl 2 equiv.Mn(OAc)3 CO2Me 1 equiv. Cu(OAc)2 O Me CO2Me HOAc 41% 60% CO2Et O H CO2Et (formal synthesis) H CO2Et Me Total Synthesis of Sesquiterpenes via Intramolecular Ketene Cycloadditions: Isocomene and !-cis- and !-trans-Bergamotenes, an Approach to Seychellene. JOC, 1988, 53, 4508-4515. O O O OEt HO2C LDA, THF reflux 1) LDA • 1) NaBH4 then CCl4 reflux TMS 1) (COCl)2 O 2) Diketene, DMAP 2) NaOH, EtOH 72% 2) TEA, PhMe 76% 78% O Reflux O O (Carroll rearrangement) O Cl TEA, PhMe reflux Li HI,PhH Me O quench work up then Li 62% O 51% Me O O Isocomene (formal synthesis) 6 steps 10% yield from commerical material O 31% 4.5% 4 The Chemistry of Barry B. Snider Baran Group Meeting Paul Krawczuk Synthesis of (-)-Reiswigin A. Assignment of Absolute and Relative Configuration. TL, 1989, 30, 2465-2468. JOC, 1990, 55, 4392-4399 HO TMS O TiCl4 1) TsOH, (CH2OH)2, PhH HO 2) OsO4, NMO, acetone 78% O DCM, -40°C 88% O H 1) NaIO4, acetone O 1 2) AcOH, piperidine PhMe O H O O 2 (L)-tert-leucine hexanes tert-butyl ester mol. sieves 94% O OH H !=(-)-Reiswigin "=ent-Reiswigin 1) H Me2AlCl DCM, 0°C 1) PCC, DCM 2) TsOH, PhH, H2O 84% O HPLC O H MgBr t-Bu-O R H R 2) MeI,HMPA workup 32% N O 4 diasteriomers R Manganese(III)-Based Oxidative Free-Radical Tandem and Triple Cyclizations. JACS, 1990, 112, 2759-2767. General Mechanism: O O CO2Me Mn(OAc)3 O O CO2Me CO2Me CO2Me Cu(OAc)2 HOAc O O CO2Me Cu(OAc)2 CO2Me -HOAc -CuOAc 86% (AcO)2Cu (14% without Cu(OAc)2) Examples: O O CO2Me CO2Me O CO2Me Mn(OAc)3 Cu(OAc)2 HOAc O CO2Me Mn(OAc)3 Cu(OAc)2 HOAc 60% H 39% O 21% CO2Me H H H 5 The Chemistry of Barry B. Snider Baran Group Meeting Paul Krawczuk Total Synthesis of (±)-Chondrillin, (±)-Plakorin, and Related Peroxy Ketals. Development of a General Route to 3,6-Dihydro-1,2-dioxin-3-ols. JACS, 1992, 114, 1790-1800. O O OMOM OMOM OAc 1) nBuLi, TMEDA 1) TsOH, MeOH hv, MeOH 1) Na2CO3, MeOH R R MeO R 2) RX 2) Pb(OAc)4, AcOH R OAc 2) TsOH, mol. sieves, PhH 73-89% 68% O al HC(OMe)3, PhH beng e s o r TsOH, reflux H , O hv, O 2DCM:Me OH O MeO H R MeO H R 41% 19:1 2:3 R hv (visible) OH OMe O O OH O O O O O MeOH, O2 MeO R O R MeO H R rose bengal 43% chondrillin H+, MeOH O O H R=C16H33 O O OMe O OMe O O Synthesis of (±)-Allocyanthin B2 and (+)-Erinacine A. JACS, 1996, 118, 7644-7645. O 1) Proton Sponge, Tf2O 1) BrMg 2) Pd(OAc)2, PPh3, CO i-Pr2EtN, MeOH 3) DIBALH 4) MnO2 69% CuBr-DMS CHO TMSCl, HMPA 2) KOt-Bu, MeI 68% OH O 1) LAH 2) MnO2 CHO 84% allocyanthin B2 87% 1) Pd(OAc)2, PPh3, CO i-Pr2EtN, MeOH 2) TEA, MeOH CO2Me 71% Oi-PrDMS 15:1 !:" 1) Me AlCl 2 CHO 2) i-PrDMSCl, imid. H 1) OsO4, KIO4 2) LHMDS, PhSeCl, H2O2 Oi-PrDMS 55% O 1) HOAc, H2O, THF H 2) DMP OTf 3) KHMDS, PhNTf2 54% H O Synthesis of 2,3-Dihydrofurans by Mn(OAc)3-Based Oxidative Cycloaddition of 2-Cyclohexenones with Alkenes. Synthesis of (±)-Conocarpan. JACS, 1997, 62, 6978-6984. O OMe O OMe 1) KOt-Bu, O O O THF, 65°C Mn(OAc)3 Mn(OAc)3 PhH, Reflux 2) LiPPh2, THF Cl PhH, Reflux 25% 77% R1 R3 25-42% R1 R3 Cl Conocarpan R2 R2 OH 6 The Chemistry of Barry B. Snider Baran Group Meeting Total Synthesis of (-)-FR901483. JACS, 1999, 127, 778-7786. 1) O O O EtOH O MeO2C O MeOPh OMe O 2) N MeOPh NOH PhH reflux O OMe 77% O O 45 psi H2, Pd/C AcOH, 24 hours 86% O 1) TsCl, DMAP, TEA 2) NaN3, DMF O O OH N HO O 5% O MeHN (-)-FR901483 N N HO PhOMe NHMe O MeHN H N H2N O HO HN OH HN NH hv, (350nm) D2O H2N 48% HN N HO HN H2N NH O NH O OH Cycloanchinopeptolide D (bis-TFA salt) OHC H2N HN H N N HO NH O N H H N O NH Ar BocN Ar NMe Boc H N O OAc KOH, MeOH, THF(1:1) 58% 0°C, 20 min H N BocHN HN BocHN NBoc O HO TFA, DCM O Anchinopeptolide D (bis-TFA salt) hv, (350nm) CD3OD trans to cis isomerization O 36% O HO N MeOPh O MeHN 16% PhOMe 2)TFA, DCM Total Synthesis of (±)-Anchinopeptolide D and (±)-Cycloanchinopeptolide D. JOC, 2000, 65, 793-800. H NBoc O NBoc O N 1) HOBt, DCC H2N BocHN N N BocHN N OH 2) DMP O H H H OAc 40% O OH O MeO2C 1) KOt-Bu, HOt-Bu MeOPh N OH O 3) H2, Pd/C, Boc2O MeOPh N 4) NaH, MeI, DMF 65% O NMe OH 1) LiBH4, THF, then Boc HCl, AcOH/H2O 92% 2) Boc2O, TEA O 3) DMP a b N MeOPh O OMe (HO)2OP O O O MeO2C 9:1 CO2Me Paul Krawczuk N HO O N H H N O NH OH O 24% other diastereomers OH 7 The Chemistry of Barry B. Snider Baran Group Meeting Paul Krawczuk Total Synthesis of (-)-Fumiquinazolines A, B, C, E, H and I. Approaches to the Synthesis of Fiscalin A. JOC, 2003, 68, 545-563. CO2Me Bu CO2Me CO2Me TrocHN CO2Me O TrocHN TrocHN HO N 1)Cbz-L-Ala-PhNO2, TrocHN Pd2(dba), P(o-tolyl)3 S KF, i-Pr2EtN OMe K2CO3, PhH, reflux O2 2) Hg(OTFA)2, KI, I2 NCbz MeOH, DCM I NCbz 64% N N N 78% N 65% H NHCbz O O O 1) NaBH4, AcOH 2) SiO2, DCM N O NH N OH O H NCbz N O 1)PPh3, Br2, TEA FmocHN Fmoc-L-Ala, EDAC, MeCN NH O 2) excess piperidine 3) MeCN reflux H2N O TrocHN NH NH O N O Fumiquinazoline A and B O H NCbz N O O H NCbz 2) EDAC, MeCN NH2 O O FmocHN CO2H EDAC, MeCN 84% O H2, Pd/C 77% OMe N N OH N O O H NCbz HCl, MeOH 61% O NH N OH N O H 56% NCbz HN O H CbzN O MeCN, HOAc, reflux 12 hours O FmocHN N O 96% PhSe Fumiquinazoline C NH O H NCbz O Fumiquinazoline E N N OH O H2, Pd/C O 1) Zn, AcOH NH O NH PhSe N 1)PPh3, Br2, TEA, 81% O N 14% 2) excess piperidine 3) MeCN, HOAc, reflux 2 hours N O O H NCbz O O 50% 8 The Chemistry of Barry B. Snider Baran Group Meeting Paul Krawczuk Synthesis of the Carbocyclic Skeleton of Abyssomicins C and D. Org. Lett. 2005, 7, 4939-4941. LiCH2PO(OMe)2, then BuLi, TESCl O OTES (MeO)2OP 85% O 1) tBuOK, 2,4-hexadienal, THF CHO 2) THF/AcOH/H2O 3) DMP 64% O O 1) Li O 2) DMP O HO O O O O O O O MeO LiCl, DMSO O O CHCl3, hydroquinone 70°C 2 days O O O O 31% MeO O MeO O O O HO Abyssomicin C A Short, Formal, Biomimetic Synthesis of (±)-Polgalolides A and B. Org. Lett. 2007, 9, 873-874. O O O O O O TEA, DCM O AcO 34% O OAc O O AcO OAc O O OAc O O AcO AcO Cs2CO3 1:1 THF/H2O O then acidify to pH 1 O O O Polygalolide B O O OMe MeO OH O O O O O O (formal synthesis) O O O O O O O O 9 The Chemistry of Barry B. Snider Baran Group Meeting Paul Krawczuk Formal Synthesis of (±)-Platensimycin. Org. Lett. 2007, 9, 1825-1828. OMe 1) K, NH3, Et2O, t-BuOH, -78°C, 15 min H O O NaBH4, EtOH 84% Br O H AIBN, n-Bu3SnH, PhH + 2) LiBr, 30 min. 3) Br Br 4) HCl, THF O H O O H 87% Br OH O O 51% (70% after HCl) 35% HCl/THF O H MnO2 HO O H O 94% H SeO2 (3 equiv.) dioxane microwave O 83% (7% enone) H Tf2O, pyr., DCM then i-PrOH then silica gel 84% O TFA/DCM then K2CO3, MeOH H L-Selectride 83% OH 90% OH OH (formal synthesis) Biomimetic Synthesis of the Tetracyclic Core of Berkelic Acid. Org. Lett. 2007, ASAP. O O 1) LDA, THF DIBALH O O O O O O HO O + 2) Dowex H , MeOH O O H unstable HO H O O MeO2C O CO2Me OH O CO2Me OH O O Berkelic Acid O C5H11 O O 24% 50% after TFA desired (thermodynamically most stable) 2) CH2N2, Et2O 3) 0.2% TFA in CDCl3, 12 hours C5H11 CO2Me OH O C5H11 CO2H OH 1) Dowex H+ MeOH O O CO2Me OH O C5H11 6% 6% after TFA O O CO2Me OH O C5H11 24% <2% after TFA C5H11 6% 0% after TFA 10 The Chemistry of Barry B. Snider Baran Group Meeting Paul Krawczuk Suggested further reading: Me HO H Cl Me H N H N H OH OHC O H N N O OH O OH O NH OH AcO HO Me H Total Synthesis of (±)-Cylindrospermopsin. JACS, 2000, 122, 5017-5024. Formal Synthesis of (±)-Guanacastepene A. JOC, 2003, 68, 1030-1042. O H Me O Total Synthesis of (±)-Leporin A. JOC, 1996, 61, 2839-2844. OH O Efficient Syntheses of Rugulosin Analogues. JOC, 2005, 70, 6863-6869. HO H N OMe OHO OH N H HO N H (CH2)10 (CH2)10 N Cl- Syntheses of Ficuseptine, Juliprosine, and Juliprosopine by Biomimetic Intramolecular Chichibabin Pyridine Syntheses Org. Lett. 2005, 7, 2715-2718. H H O H O H C7H15 Total Synthesis of (±)-DeoxypenostatinA JOC, 1999, 64, 1088-1089.