ACIEE 2004 Baran Group Meeting
advertisement
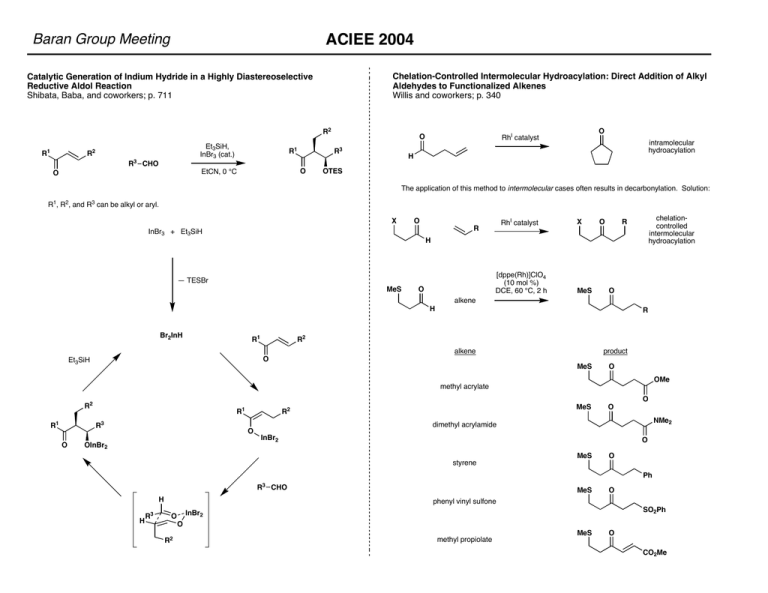
ACIEE 2004 Baran Group Meeting Chelation-Controlled Intermolecular Hydroacylation: Direct Addition of Alkyl Aldehydes to Functionalized Alkenes Willis and coworkers; p. 340 Catalytic Generation of Indium Hydride in a Highly Diastereoselective Reductive Aldol Reaction Shibata, Baba, and coworkers; p. 711 R2 R1 Et3SiH, InBr3 (cat.) R2 R3 CHO R1 O EtCN, 0 °C O R3 O RhI catalyst O intramolecular hydroacylation H OTES The application of this method to intermolecular cases often results in decarbonylation. Solution: R1, R2, and R3 can be alkyl or aryl. X O RhI catalyst R InBr3 + Et3SiH X O chelationcontrolled intermolecular hydroacylation R H [dppe(Rh)]ClO4 (10 mol %) DCE, 60 °C, 2 h — TESBr MeS O MeS O alkene H Br2InH R1 R R2 alkene O Et3SiH product MeS O OMe methyl acrylate O R2 R1 R1 R3 O O OInBr2 MeS R2 O NMe2 dimethyl acrylamide InBr2 O styrene MeS O Ph R3 CHO H H R3 MeS O phenyl vinyl sulfone SO2Ph InBr2 O O R2 methyl propiolate MeS O CO2Me ACIEE 2004 Baran Group Meeting A Mild and Efficient Synthesis of Oxindoles: Progress Towards the Synthesis of Welwiindolinone A Isonitrle Wood and coworkers; p. 1270 Me Efficient Asymmetric Hydrogenation of Pyridines Glorious and coworkers; p. 2850 Cl R4 R5 H Me Me CN R6 R3 N Welwitindolinone A Isonitrile S S S O cat. DBU; Cl2CO, Et3N H Me Me H O S Me O SmI2, LiCl, — 78 °C Me S H Me Me O 71% H OCN NH N H •HCl i-Pr The starting materials for this transformation are made from the appropriate 2-halopyridine and the Evans oxazolidinone using K2CO3 and CuI. S H O R3 O R6 O O N H R5 N H O i-Pr R4 1) H2 (100 bar), AcOH, catalyst 2) aq. HCl O O Running the reaction in acidic medium is required for several reasons: 1) protonated pyridines are more susceptible to reduction than are free pyridines; 2) the prodcuts could poison the hydrogenation catalyst as the free amine; 3) protonation locks the conformation of the substrate as shown allowing for effective enantiocontrol. The aminal produced during hydrogenation decomposes to an iminium, which exists in equilibrium with the eneiminium species, scrambling the stereochemistry at C3. N H HN Copper(I)-Catalyzed Asymmetric Hydrosilylations of Imines at Ambient Temperature Lipshutz and coworker; p. 2228. Asymmetric Catalytic Coupling of Organoboranes, Alkynes, and Imines with a Removable (Trialkylsilyloxy)ethyl Group— Direct Access to Enantiomerically Pure Primary Allylic Amines Jamison and coworker p. 3941 TBSO O N P xylyl xylyl O 6 mol % CuCl, 6 mol % NaOMe, 6 mol % R-(—)-DTBM-SEGPHOS, 3 eq. TMDS, 3.3 eq. t-BuOH, r.t. H N P xylyl R1 TBSO xylyl R NH R1 H R Et 5 mol % [Ni(cod)2]/L* Et3B, (3 eq) N R R R2 R2 (2 eq) X X t-Bu OMe O Me O xylyl = O P t-Bu P t-Bu 2 Me O OMe t-Bu 2 8 examples with ee's > 94%; in all cases examined the R stereoisomer was formed; R group can be alkyl or a benzofused ring; EWG's and EDG's on the aromatic ring are tolerated Alkyne component must be symmetric unless either R1 or R2 is an aryl group. R was limited to aryl or saturated rings in the present study. P L*: Ph Ar Fe Ar = 2-(i-Pr)Ph ee's range from 70% to 89% ACIEE 2004 Baran Group Meeting Stereospecific and Stereodivergent Constrcution of Quaternary Carbon Centers through Switchable Directed/Nondirected Allylic Substitution and Iterative Deoxypropionate Synthesis Based on a Copper-mediated Directed Allylic Substitution Breit and coworkers; p. 3786 and 3790 Rh-Catalyzed Amination of Ethereal Ca—H Bonds: A Versatile Strategy for the Synthesis of Complex Amines DuBois and coworkers; p. 4349 O HN O S O RO O CuBr R4MgX R2 R1 R4 R2 Ph2P M R3 R4 R3 O H R1 * R3 H CuI Ph2P R2 R4 R3 R1 R3 O R2 O H R1 O Nu R2 R1 Formation of 1,4-Diketones by Aerobic Oxidative C—C Coupling of Styrene with 1,3-Dicarbonyl Compounds Christoffers and coworkers; p. 6547 R4M R2 O S Lewis acids: BF3•Et2O, Sc(OTf)3 nucleophiles: allyltrimethylsilane, thioketene acetals, enol silyl ethers The diastereoselectivity observed is highly dependent on pre-existent stereochemistry. O PPh2 O R1 O O R2 O R2 HN R1 PPh2 O O Lewis acid, carbon nucleophile R1 H O 1) air, CeCl3•7H2O (10 mol %), i-PrOH, styrene, r.t. 2) AcCl, py O Essentailly complete transfer of chirality (99%) is observed in most cases. Yiields generally range from good to excellent. O Ph OEt * R3 M R4 O CO2Et Enantioselective Synthesis of Cyclopropanes by Aldehyde Homologation Taylor and coworker; p. 6671 OH Me BrMg CuBr•DMS (0.5 equiv) i-Pr OPMB CuBr•DMS (0.5 equiv) O(o-DPPB) Et N O Me R Me Et Me Me OPMB Et dr = 97:3 83% O Me Me Tf2O, 2,6-lut., —78 to —50 °C Also applicable to N-alkyl, N-Boc crotylamines. OPMB Et N(i-Pr)2 O i-Pr O(o-DPPB) Me Me n-BuLi, (–)-sparteine; Ti(Oi-Pr)4, RCHO O dr = 99:1 80% Diastereoselectivity for the oxygen manifold is not complete due to equilibration after product formation; this is not a problem with the nitrogen analogue. Yields are generally high. Iterations can be carried out by ozonolysis (92% or greater), iodination (92% or greater), and metal halogen exchange followed by exposure to the conditions shown above. Me H R CHO H ACIEE 2004 Baran Group Meeting Asymmetric Total Synthesis of (—)-Nakadomarin A Nishida and coworkers; p. 2020 A Formal Total Synthesis of (+)-Pinnatoxin A Inoue, Hirama, and coworkers; p. 6505 Me H Me O HN N N H CO2 O O Me Total Synthesis of Apoptolidin Koert and coworkers; p. 4597 OH HO Me OH O O OH O Me Me O MeO Total Synthesis of Apoptolidinone Sulikowski and coworkers; p. 6673 Me Me Me OH O Me O H Me OH MeO HO O H Me HO OMe Me Me O O Me Me OH O O HO OH OMe Me Me OH O Me O Me H OH MeO HO An Effecient Total Synthesis of Optically Active Tetrodotoxin Isobe and coworkers; p. 4782 O Me H2N O O OHO OH OH OMe Me OH HO H N N H HO H OH ACIEE 2004 Baran Group Meeting Structural Revision and Total Synthesis of Azaspiracid-1, Part 1: Intelligence Gathering and Tentative Proposal Nicolaou, Satake, and coworkers; p. 4312 Structural Revision and Total Synthesis of Azaspiracid-1, Part 2: Definition of the ABCD Domain and Total Synthesis Nicolaou and coworkers; p. 4318 Total Synthesis of (±)-Nominine, a Heptacyclic Hetisine-Type Aconite Alkaloid Muratake and Natsume; p. 4646 N H O H O H OH O Me H O HO A Concise Asymmetric Synthesis of the Marine Hepatotoxin 7-Epicylindrospermin Williams and coworker; p. 2930 H H NH O Me H Me Me O HO2C OH H Me O O Me H O3SO Me Me H HO H H N NH N NH Total Synthesis and Configurational Assignment of (—)-Dictyostatin, a MicrotubuleStabilizing Macrolide of Marine Sponge Origin Paterson and coworkers; p. 4629 Total Synthesis of (—)-Dictyostatin: Confirmation of Relative and Absolute Configurations Curran and coworkers; p. 4634 O NH OH Synthesis of the C-1027 Chromophore Framework through Atropselective Macrolactonization Inoue, Hirama, and coworkers; p. 6500 H2N Boc2N HO Me OH O MOMO HO Me Me Me Me Me O O Cl O OH OTES (synthesized) O Cl OMe O HN OPMB OH OTES MOMO OH O O O O O Me O OH O Me OH NMe2 (final target) O ACIEE 2004 Baran Group Meeting Enantioselective Total Synthesis of Batzelladine A Nagasawa and coworkers; p. 1559 A Highly Efficient Synthesis of Lamellarins K and L by the Michael Addition/ Ring-Closure Reaction of Benzyldihydroisoquinoline Derivatives with Ethoxycarbonyl-b-nitrostyrenes Ruchirawat and coworkers; p. 866 NH2 O O N H NH2 O H O N NH N N Me NH2 O H O X N H N H (CH2)8Me X X NO2 EtO2C TIPSO OpMB EtO2C TIPSO CO2Et H 7 N X H N N O X N NaHCO3, MeCN, 70 °C CO2Et X OpMB 7 O X X X 1. LAH 2. CsF H 1) TBSCl 2) Barton deoxygenation CH2OTBS H N N 7 1. Pd(OH)2, H2 2. A, HgCl2, Et3N H O O BocHN NBoc HO OTBS Me O R = H: Eriolanin R = Me: Eriolangin O H NHBoc NBoc OH SMe OH 7 N OpMB 7 O A: OTBS H Enantioselective Total Synthesis of the Highly Oxygenated 1, 10-Eudesmanolides Eriolanin and Eriolangin Metz and coworkers; p. 5991 CH2OH H OpMB O HO OpMB PPh3, DEAD 7 N R OpMB Me NBoc NBoc i) MeLi, —78 °C ii) LiCH2CH2Si(Me)2Ph, —78 to —20 °C iii) ICH2MgCl, —78 to 23 °C O O O OH HO S O Me SiR3 Me ACIEE 2004 Baran Group Meeting Synthesis of the Furanosteroidal Antibiotic Viridin Sorensen and coworkers; p. 1998 An Efficient Synthesis of Lactacystin b-Lactone Donohoe and coworkers; p. 2293 O OH MeO Me NH Me O Me O O 1. Li, DBB, ((MeO)CH2CH2)2NH, MgBr2, isobutyraldehyde NBoc 2. Ac2O, py, DMAP O OTBS I 1. cat. OsO4, NMO 2. PPh3, DBAD, MeI NBoc Me HO EtO2C EtO2C CO2Et Me AcO OTBS i-Pr2EtN, xylenes, reflux; DDQ, r.t. OH O O Me NBoc Me AcO Me 1. cat. InCl3, NaBH4 2. TESCl, imid, DMAP Me Me OTES OTES O O O 1. TFA 2. aq. NaOH Me 3. BOPCl, Et3N Me NH Me TMS TMS CsF, allyl Br OTBS OTBS mesitylene, 165 °C Me TESO EtO2C OH O O Me O NBoc Me AcO 1. RuCl3, NaIO4 2. HF•py 3. LDA, HMPA, MeI TESO EtO2C AcO Me Stereoselective Total Synthesis of (—)-Borrelidin Theodorakis and coworkers; p. 3947 Me Me Me Me OH O O O OH Me O RCM NC O O CO2H OTBS Me O O O 1. n-Bu4SnH, Mo(CO)3(t-BuNC)3 2. I2 3. n-Bu4SnCN, Pd(PPh3)4, CuI R R O NC R R NBoc Me Me ACIEE 2004 Baran Group Meeting Fully Stereocontrolled Total Syntheses of (—)-Cylindricine C and (—)-2Epicylindricine C: A Departure in Sulfonamide Chemistry Ciufolini and coworkers; p. 4336 H O H Stereocontrolled Total Synthesis of (+)-Streptazolin by a Palladium-Catalyzed Reductive Diyne Cyclization Trost and coworkers; p. 4327 Me O OH n-C6H13 N N n-C6H13 N OH OH H O O Me Me TsO Pd2dba3•CHCl3, HCO2H, Et3SiH TBDPSO MeO2S OH HN MeO2S 1. PhI(OAc)2 2. TBDPSCl, imid TsO OTBS H HN HN N OTBS O H O O O O HO K(TMS)2N, —100 °C TBDPSO O2 S N H Enantioselective Total Synthesis of (+)-Milnamide A and Evidence of Its Autooxidation to (+)-Milnamide D Molinski and coworkers; p. 5951 TBDPSO O2 S N H 1. PhSH, BF3•Et2O 2. Raney Ni Me Me O H N Me NMe Me N H Me Me Me N O Me CO2H Me Me O 1. t-BuLi, 1-octene oxide, BF3•Et2O 2. DMP n-C6H13 O TBDPSO O2 S N H 1. DBU; bis(pinacolyl) diboronate, CuCl, KOAc 2. Na(CN)BH3, AcOH; H2O2, aq. NaOH 3. DMP 4. TBAF Me Me O H Me Me MeN O SeO2, CHCl3, reflux O N Me HO n-C6H13 OH N Ph N N O Ph ACIEE 2004 Baran Group Meeting Total Synthesis of Thiostrepton, Part 1: Construction of the Dehydropiperidine/ Thiazoline-Containing Macrocycle Nicolaou and coworkers; p. 5087 Total Synthesis of Thiostrepton, Part 2: Construction of the Quinaldic Acid Macrocycle and Final Stages of the Synthesis Nicolaou and coworkers; p. 5092 O S N O H N N H Concise Total Synthesis and Structure Assignment of TAN-1085 Suzuki and coworkers; p. 3167 O HO OH NH2 O OH O N N H S Me S O Me Me O HN OH N O H N N S HO N H Et OMOM O Me Me S Me OH OH S N R HN N S N COCl H2N S Et3N H N EtO2C N3 Me BocN O Me Me EtO2C Me O Me Me OMOM —78 °C, SmI2; BzCl BnO N BocN BnO MeO CHO CHO OBz H S OMOM CO2Et N N N S BnO MeO S CO2Et OTBS 1. desilylation 2. Swern [O] R Me N OBn S OMe OMe S Me S MeO then MeOTf R N N O Me OBn OMe OMe N N BocN O N S Me S N3 N Ag2CO3, DBU, BnNH2 OTBDPS BnO OTBS R S OMOM Li O N HN OTBDPS BnO OH S Me O N O Me O Me O HN EtO2C N H O Me O HN NH HO H N N Me N O O OMe OH BnO OMe





