Modifying and Checking 2 2.1 Modifying the chosen procedure
advertisement

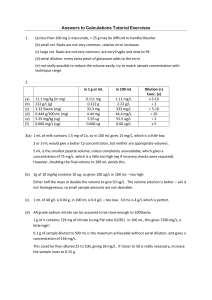
Modifying and Checking 2 2.1 Modifying the chosen procedure Having decided on the preferred method, it is then a matter of seeing how well it fits your particular task: that is, how much do you have to change before actually seeing how well it works. If you were really lucky in your search, the amount of modifications will be small. It is rare that any method described in the literature will work without any modifications for a specific application. Some of these modifications will include: changes in sample amount to fix concentration problems at the analytical step chemical problems, such as pH correction more efficient ways of doing certain steps (see section 2.3) inaccuracies due to matrix interference or other reasons, found the in the checking procedures – these will be the hardest to fix, and may require further literature research or trial-and-error experimentation The most likely change that will need to be made is in getting the right amount of sample to meet the concentration range of the analytical technique. This means calculating how much sample to weigh out (if a solid) and what dilution scheme to use (for liquids and solutions). There are various possible answers to this, but in general try to aim your final analysed solution towards the lower middle part of the standard range. This allows for variations in concentration, but also for validation procedures such as recovery checks. There are many ways to approach this and many answers, and it can be difficult to work out where to start, even if you know where you want to finish. After all, there are many combinations of masses, pipettes and volumetric flasks that could get you to a suitable concentration for analysis. We suggest the following way of working it out – it uses simple numbers and proportions, no formulas – but does require that you have an approximate idea of the expected concentration. 1g 1 mL Suggested method of determining sample amount and necessary dilution 1. Start with 1 g or 1 mL of sample. Calculate how much analyte is in this amount. Work this out in the unit specified in the standard range, eg if the range is in mg/L, use mg. 2. Determine the concentration (in mg/L or g/L) if the 1 g/mL of sample is dissolved in a 100 mL volumetric flask. This is very easy – just multiply by 10! (per 100 mL up to per L). 3. Identify how much dilution or concentration is required to get the analyte level to the required range. Use simple proportions (eg 2x, ÷5). 4. Work out how this can be achieved in practice by changing sample amounts or dilution (via pipette/vol. flask). Points to consider 1. The “standard” range for titrations is 15-30 mL of the titrant. 2. Sample masses should not be less than 100 mg or >25 g. 3. Pipette volumes should be between 5 and 50 mL. 4. Volumetric flasks should be between 50 and 500 mL. 2. Modifying & Checking 5. Small decreases in concentration are done by reducing the sample amount or increasing the volumetric flask volume, larger decreases by dilution. Avoid serial dilutions where possible – try a less sensitive technique. 6. Increases in concentration are best done by increasing the sample amount (see pt 2). If this is not possible, a less sensitive technique is probably the better option. EXERCISE 2.1 Explain the reason for Points 2-4. EXERCISE 2.2 1. How much analyte in the specified unit in 1 g or mL of the sample? What is the concentration when in 100 mL? (a) 11.1 mg/kg (in mg) (b) 222 g/L (g) (c) 3.33 %w/w (mg) (d) 0.444 g/100 mL (mg) (e) 5.55 mg/kg (ug) (f) 0.666 mg/L (ug) 2. What dilution (or concentration) would be appropriate for the samples in 1(a-f) given the following standard ranges? (a) 5-20 mg/L (b) 0.5-2 g/L (c) 10-100 mg/L (d) 1-5 mg/L (e) 10-50 ug/L (f) 10-50 ug/L Project p2 2. Modifying & Checking EXAMPLE 2.2 (a) The fluoride in toothpaste is to be analysed by ISE. The range of standards for the technique is 5-100 mg/L. The sample is expected to be 0.1%w/w fluoride. What is a suitable sample amount and dilution? 0.1%w/w means 0.1 g or 100 mg fluoride per 100 g of toothpaste. Therefore 1 g of toothpaste contains 1 mg of fluoride. 1 g of toothpaste dissolved in 100 mL means 1 mg F/100 mL = 10 mg/L. This is at the bottom end of the standard range, so it would be OK, but really a little low, so we need more sample. 2 g will give 20 mg/L, which is better. 5 g will give 50 mg/L, which would be as high as you would go. (b) Potassium in fertiliser: 12%w/w; standards 5-50 mg/L 12%w/w = 12 g K/100 g fertiliser 1 g fertiliser contains 12/100 g of K = 0.12 g = 120 mg/L. 1 g fertiliser in 100 mL vol. flask = 120 mg/100 mL = 1200 mg/L. Target concentration = 25 mg/L. Try different dilutions: 10x = 120, 20x = 60, 50x = 24. Answer 1. 1 g of sample in 100 mL vol. flask, then dilute 5 mL to 250 mL. Answer 2. 0.1 g sample in 500 mL vol. flask also gives same result => better method if sample is homogeneous. (c) Protein in milk: 4 g/100 mL; sample amount containing around 100 mg of protein. 4 g/100 mL = 4000 mg/100 mL. 1 mL of milk contains 40 mg of protein. 2.5 mL of milk contains 100 mg. EXERCISE 2.3 Determine a suitable sample amount and/or dilution for the following: (a) The calcium concentration in milk is typically 0.15 g/100 mL. The standards for the ICP are 1-100 mg/L. Project p3 2. Modifying & Checking EXERCISE 2.3 (CONT’D) (b) A soil sample has an expected lead level of 10 mg/kg. The analytical procedure requires a solution with a concentration between 25-100 ug/L. (c) The expected acid concentration of vinegar is 40 g/L. The GC standards are 1-10 g/L. (d) A purity check on AR grade sodium nitrate requires the preparation of a solution of approximately 50 mg/L nitrate. FWs: Na 23, O 16, N 14 EXERCISE 2.4 Determine appropriate sample amount/dilution schemes for the following. (a) Lead content in soil, where the expected level is 100 ug/kg and the range of standards is 10-50 ug/L. (b) Benzoic acid in soft drinks at concentrations around 300 mg/L by UV-VIS spectroscopy (standards 20-100 mg/L). (c) Paracetamol in tablets (500 mg/tablet) – the standards are 2-10 mg/L. (d) Iron in steel (around 95%w/w), with standards of 2.5-10 mg/L. (e) Analysis of Weetbix for sodium content, where the expected level is 290 mg/100 g and the range of standards is 1-20 mg/L. (f) Phosphorous in copper: expected 1%w/w, standards 5-20 mg/L. (g) Vitamin C in capsicums: expected 200 mg/100 g, sample amount 5 mg Project p4 2. Modifying & Checking What if you don’t know even an approximate concentration? In environmental testing, this will often be the case. You can make an educated guess based on what information you do have, or you can use the sample amount/dilutions suggested in the literature method. When you run the sample through the procedure, you will then determine how far out you are with the concentration. If you get a low concentration, then you obviously adjust the sample amount upwards. if you get a high value when analysed, do not simply dilute the final solution again as part of the method. The decrease in sample amount should occur before the analysis step, because dilution afterwards can cause an error for reasons such the sample matrix no longer matching the reagent blank or the concentration of colour forming reagent is diluted as well as the analyte. 2.2 Checking the method There is no guarantee that a published method will work absolutely perfectly. This can be because the work has not been fully checked, or more commonly because your sample has particular characteristics, which cause problems. Therefore, before a method can be used for general purpose analysis, it must be checked for its: ease of performance – this comes down to experience of the technician, who should be able to recognise better ways of doing certain steps in the procedure precision – you must also run the procedure a number of times (at least five) on the same sample to assess its consistency, and accuracy – the most important part of the checking process and in some ways the most difficult to verify (or validate); it is not ever possible to state that the actual analysis result for an unknown sample is correct; all you can do, using a combination of approaches described below, is as good as it can be Blanks With any procedure, a number of reagents will be added to the sample, e.g. dilution solvent, acid for decomposition, colour forming reagents, pH buffers etc. It is essential, particularly for trace and ultratrace analysis, that these reagents are checked for their purity by the use of a blank, which contains all reagents in exactly the same quantities as the prepared sample. For example, the decomposition of foodstuffs is often performed by boiling sulfuric acid. If an analysis of iron in meat was performed, using this decomposition method, a 0.001% Fe impurity in the 30 mL of sulfuric acid would add 0.3 mg of iron to the analysis. This might increase the Fe levels in the sample solution by more than 10%. In a spectroscopic analysis, this would serve as the zero, and any absorbance/emission by impurities in the blank would be automatically subtracted. In other techniques which don’t involve a zeroing step, the blank is analysed in the same way as the sample, and any reading subtracted manually, e.g. the Karl Fischer moisture determination would need careful analysis of the solvent/reagent system to determine its moisture content (as mL of titrant) which can then be subtracted form the mL of titrant for the sample. Standard samples If the method development is being done to improve the efficiency of analysis of an ongoing sample type, then it may be that the laboratory has a stock of samples of similar nature (i.e. matrix) whose composition is already known. These are often called Certified Reference Materials (CRMs). In this case, the standard sample can be analysed using the new method, and the result checked with the accepted value. This is perhaps the best checking method, but is not always possible to obtain such a material. Using another method For similar reasons to those for the use of standard samples, checking the answer from the new method with that obtained from another accepted (perhaps less convenient or more expensive) method is a satisfactory checking method. It may be that the checking analysis is performed by an outside laboratory (for a charge obviously), because this laboratory has access to equipment that yours doesn’t. Project p5 2. Modifying & Checking Analytical procedures The methods of compensating for errors caused by matrix or technique - matrix-matched standards, standard addition and internal standards - should be familiar to you by now, and must be considered when designing your procedures. Standard addition is time-consuming, because each sample requires its own set of “standards”, and therefore, should be avoided unless matrix interference is a problem or you are working at ug/L levels. However, you should consider a standard addition analysis as a check on a conventional calibration graph-based method, just to see whether the two results are different. Recovery checks This requires the addition of a known amount of analyte (known as a spike) to the sample, which is then analysed as normal. The increase in concentration as analysed is then compared to how much was due to the sample and how much was added. If a procedure works perfectly, then 100% of the added analyte will be recovered. For example, if your analysed portion of sample contained 5 mg of analyte, and another 5 mg was added as a spike, you would expect when the spiked sample was analysed, it would contain 10 mg. If less (or more) is recovered than was put in, then something is going wrong in the procedure. The key conclusion to make here is that the percent recovered is probably a measure of how accurate the answer for your sample is. If only 50% of the added analyte is recovered, it suggest that 50% of the analyte in the sample is being missed as well. However, you don’t then adjust the sample answer accordingly, just report both results. The recovery check process is similar in performance to standard addition, but is done for totally different reasons. Standard addition is done to determine the sample concentration (it is an analysis procedure), while the recovery check requires that you know the concentration in the sample (it is check on the analytical procedure). In general, a recovery of between 90-110% is considered perfectly reasonable. Recovery check calculations The %recovery is calculated as below. amount in spikedsample amount in sample % re cov ery 100 x amount in spike Amount can refer to mass, volume or concentration. For the rest of this, SPS means amount in spiked sample, S amount in sample and SP amount in spike. The final calculation itself could not be much easier. However, getting the three numbers to plug into the equation is less easy, because it is easy to put the wrong number in when there are so many numbers to choose from in a typical multi-step analysis. Remember the two rules below and it will help you avoid some of the common errors. 1. All three values must be in exactly the same unit, eg mg, g/L, %w/w 2. All three values must be from the same stage of the analysis, eg the original sample, the first solution, the analysed solution Project p6 2. Modifying & Checking One way to start these calculations, especially when you are learning, is to identify which numbers belong to which solution: sample, spike, spiked sample, or the procedure itself (details such as dilution volumes). You will find that no number should belong to more than one of these categories. However, you may not feel that this method is necessary and that you can do them a different way that you feel more comfortable with. That is perfectly fine. There is not one correct way to do all recovery calculations, but there is only one correct answer. EXAMPLE 2.3 10 mL samples of river water are diluted to 50, and found to contain an average of 2 mg/L of Na. Another 10 mL aliquot of the water is spiked with 1 mL of 50 mg/L Na, made up to 50 mL and then analysed. It was found to contain 2.9 mg/L. S SP SPS Procedure 2 mg/L 1 mL of 50 mg/L 2.9 mg/L 10:50 Fairly obviously, the correct unit to choose is mg/L because it is the only one in common. This procedure has only two stages: the original river water and the diluted solution which is analysed. Which stage do these mg/L numbers belong to? S : 2 – diluted SP: 50 – neither (it is a standard in another bottle) SPS: 2.9 – diluted It would seem simplest to work out what the mg/L of SP in the diluted solution is, because that completes the set of three. This is simply the dilution equation you know very well: C1V1 = C2V2. 50 x 1 = ? x 50, so SP mg/L = 1 2 .9 2 %RC 100 x = 90% 1 EXAMPLE 2.4 Potato chips are analysed and found to contain 5.0%w/w of Na. A 1 g sample is spiked with 5 mL of 5000 mg/L Na and analysed. It is found to contain 70 mg of Na. S SP SPS Procedure A little tougher now as every number has a different unit. However, there is only one stage. Project p7 2. Modifying & Checking Approach 1 – mass based Use mg as the common unit, so SPS is already done. For S, how many mg are in 1 g of 5%w/w? For SP, how many mg are in 5 mL of 5000 mg/L? %RC 100 x = Approach 2 - concentration based Use %w/w as the common unit, so S is already done. For SPS, what is the % of 70 mg in 1 g? For SP, which has 25 mg, what is the %? = %RC 100 x Same answer – is one approach better? No. EXERCISE 2.5 (a) 10 mL of sample (known concentration 100 mg/L Ca) is spiked with 500 uL of 1000 mg/L Ca and diluted to 250 mL. This solution, when analysed, has a concentration of 6.1 mg/L Ca. S SP SPS Procedure Project p8 2. Modifying & Checking EXERCISE 2.4 (CONT’D) (b) 5 g of soil (known concentration 50 mg/kg lead) is spiked with 5 mL of 50 mg/L Pb, and made to 100 mL. The concentration of this solution is 4.4 mg/L. S SP SPS Procedure (c) A beer sample is diluted 1:1 and analysed for its sodium content by flame photometry, using simple Na standards. The concentration of the beer is found to be 50 mg/L. A 50 mL aliquot of the beer is spiked with 2 mL of 1000 mg/L Na, made up to 100 mL and this solution found to contain 40 mg/L Na. S SP SPS Procedure Project p9 2. Modifying & Checking EXERCISE 2.6 Now for some real values, rather than nice simple whole numbers. (a) The concentration of Vitamin C in an orange juice sample was determined by HPLC to be 41.2 mg/100 mL. Another 25 mL aliquot of the sample was spiked with 10 mL of 1000 mg/L Vitamin C standard and the solution made up to 50 mL. The spiked sample was analysed under the same conditions and found to contain 398 mg/L. (b) The ethanol content of a wine sample was determined to be 12.3 %v/v. A 5 mL aliquot of the wine was spiked with 200 uL of pure ethanol. When analysed it was found to contain 16.5%v/v. Assume no volume change when the spike is added. (c) The sodium content of a butter sample was determined to be 475 mg/100 g. A recovery check was prepared by weighing out 2.4376 g of sample, dispersing it in hot water, adding a 5 mL aliquot of 1000 mg/L sodium and making up to 100 mL. A 10 mL aliquot was then diluted to 100 mL for analysis. The sodium content of this solution was 15.9 mg/L. (d) A 5 mL aliquot of bitter lemon (quinine concentration 27.4 mg/L) is spiked with 200 uL of 200 mg/L quinine standard and diluted to 25 mL. A 20 mL aliquot of this solution is determined to contain 145.9 ug of quinine. (e) A sample toothpaste is analysed for its fluoride content and found to contain 0.115 %w/w. A 5.0762 g sample is spiked with 5 mL of 500 mg/L fluoride and dissolved in 100 mL of solution. A 25 mL aliquot is diluted to 100 mL and analysed: it contains 21.6 mg/L. (f) A soluble fertiliser is analysed for its nitrate content, and found to have 10.1% w/w. A 0.5682 g sample is dissolved in water, 5 mL of 5 g/L nitrate added and the mixture made to 500 mL. A 10 mL aliquot is diluted to 50 mL, and this solution is found to contain 32.4 mg/L. (g) A 1.3825 g sample of peanuts (2.69%w/w K) is spiked with 20 mL of 1000 mg/L K, ashed and dissolved up to 100 mL. A 10 mL aliquot is diluted to 100 mL, analysed and found to contain 52.3 mg/L. (h) 25 mL of lemonade (385 mg/L benzoic acid) is spiked with 5 mL of 1000 mg/L benzoic acid and made up to 100 mL. When analysed, this solution has a concentration of 138 mg/L. (i) 2.5346 g of powdered milk (395 ug/kg Pb) is spiked with 5 mL of 100 ug/L Pb, ashed and dissolved in 25 mL. This solution is analysed and found to have a lead concentration of 62.3 ug/L. (j) 5 mL of milk (Ca 147 mg/100 mg/L) is spiked with 5 mL of 1000 mg/L Ca, ashed, dissolved in 100 mL, and then diluted 5 mL to 100 mL for analysis. The analysed concentration is 6.21 mg/L. (k) 0.2387 g of fertiliser (3.18 %w/w P) is spiked with 5 mL of 1000 mg/L P, and dissolved in 250 mL. The analysed concentration is 44.8 mg/L. (l) 9.6571 g of honey (24.2 %w/w water) is spiked with 1.0583 g of water, and dissolved in 100 mL. The analysed concentration is 31.8 g/L. Project p10 2. Modifying & Checking Addition of the analyte It is important that the spike of analyte be in a chemical form as similar as possible to that existing in the sample. If you were analysing the salt content in butter, then add the spike as NaCl. If you were analysing calcium in milk, where the calcium is bound up in the milk protein, it becomes harder to duplicate, but you might try adding the calcium as a complex (maybe EDTA??). The amount of analyte added in the spike is not just whatever comes to hand, and should be: enough to make a measurable difference to the analysis value (eg absorbance, titre volume) not so much that the spiked sample moves outside the working range of the procedure, and therefore requires an extra dilution an convenient and accurate amount In general, the amount of spike added should be about 50% of the amount of analyte in the aliquot/mass of sample required for the analysis. EXERCISE 2.7 Why would an extra dilution of a recovery check (because too much was added) be undesirable? Where small amounts (mg or ug) must be added, this will require an aliquot of a stock solution. 1 mg is contained in 1 mL of 1000 mg/L solution 1 ug is contained in 1 mL of 1000 ug/L solution EXAMPLE 2.5 A sample of meat is analysed for its iron content by acid decomposition then AAS, using simple Fe standards. A 10 g sample of meat is found to contain 5 mg of Fe from the calibration graph. Devise a suitable recovery check. 25-100% of the analyte amount is between 1.25 and 5 mg. This should be added to the meat before any preparation steps are undertaken, unless you are wanting to check only the later parts of the procedure. It cannot be done directly because of the small mass, so an aliquot of a standard solution should be used, e.g. 5 mL of 1000 mg/L = 5 mg. Project p11 2. Modifying & Checking EXERCISE 2.8 (a) A 10 mL aliquot of milk is wet ashed and diluted to 100 mL. A 20 mL aliquot of this solution is further diluted to 100 mL. This solution is analysed for its calcium content by ICP, and found to be 22.7 mg/L. (i) What is the concentration of calcium in the milk? (ii) What mass of calcium is contained in the original 10 mL aliquot of milk? (iii) What would be an appropriate amount of recovery spike? (iv) How could this be added? (b) The lead content of wine was analysed by anodic stripping voltammetry. The method required a 20 mL aliquot of the juice to be diluted to 100 mL and a 25 mL aliquot of this measured. This was found to contain 0.39 ug of the analyte. (i) What is the concentration of lead in the wine? (ii) What mass of lead is contained in the original 20 mL aliquot of wine? (iii) What would be an appropriate amount of recovery spike? (iv) How could this be added? Project p12 2. Modifying & Checking EXERCISE 2.8 (CONT’D) (c) The chloride level extracted from a soil sample was found to be 50 mg/100 g. If the method calls for a sample size of 10 g of soil, suggest an appropriate recovery check method. (d) A 5 mL aliquot of vinegar (40 g/L ethanoic acid) is diluted and titrated. Suggest an appropriate recovery check method. In principle, the spike can be added at any step in the procedure, but will only test the accuracy of the steps after the addition. In general, you would start by checking the entire procedure, so the spike must be added to the measured amount of sample before any preparation is carried out. If the recovery on the entire procedure is poor, then you can spike at different stages to try to find where the problem is occurring. EXERCISE 2.9 A comprehensive recovery check program is performed on a complex procedure, and the results are as follows. Identify where the problem is occurring. Spike added sample before ashing after drying after wet ashing with addition of reagents before analysis in ICP Project % recovery 75 78 94 89 90 p13 2. Modifying & Checking 2.3 Improvements Most analytical procedures will involve heating, dissolving, reagents of various types and concentrations etc. How do you know what is best for your sample in terms of time/temperature/ concentration? You don’t! You can only determine these by trial-and-error. What you are doing here is improving the procedure, rather than getting it to work properly. However, what you must ensure is that in achieving a more efficient process, you don’t lose accuracy/precision. Therefore, make sure you have a sample whose concentration from the “standard” method is known, before you start tinkering around. EXERCISE 2.10 The procedure for determining the moisture content of bread is as follows: 1. 2. 3. 4. 5. 6. 7. Take one slice from the end of the loaf and one from the middle. Break the slices into 1-2 cm pieces and combine. Weigh a pre-dried large evaporating basin. Weigh accurately about 10 g of the pieces into the basin. Place in a 105C oven for 2 hours. Cool in a desiccator for 10 minutes. Re-weigh. What modifications to this procedure could be tried to improve its efficiency? What You Need To be Able To Do adapt procedures to fit your sample requirements describe and use methods check the accuracy of your procedure modify the procedure to improve its efficiency/accuracy Project p14