Document 12831750
advertisement
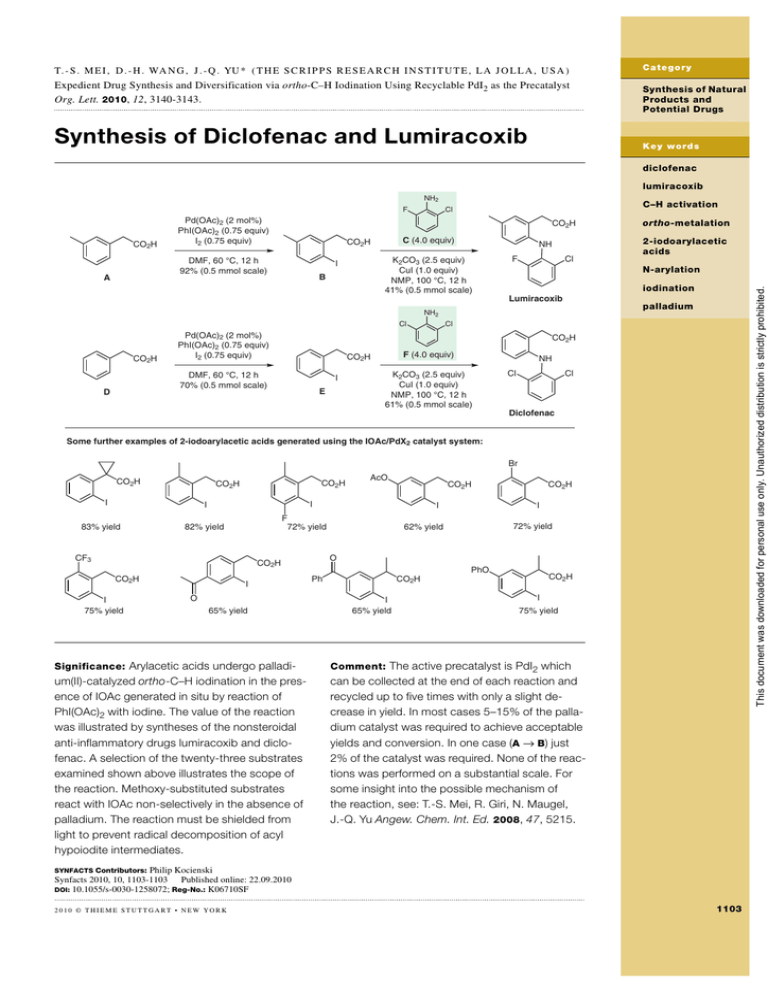
T. - S . M E I , D . - H . WA N G , J . - Q . YU * ( T H E S C R I P P S R E S E A R C H I N S T I T U T E , L A J O L L A , U S A ) Expedient Drug Synthesis and Diversification via ortho-C–H Iodination Using Recyclable PdI2 as the Precatalyst Org. Lett. 2010, 12, 3140-3143. Synthesis of Diclofenac and Lumiracoxib C a te go r y Synthesis of Natural Products and Potential Drugs K e y w ord s diclofenac lumiracoxib NH2 CO2H CO2H C (4.0 equiv) CO2H DMF, 60 °C, 12 h 92% (0.5 mmol scale) A C–H activation Cl NH K2CO3 (2.5 equiv) CuI (1.0 equiv) NMP, 100 °C, 12 h 41% (0.5 mmol scale) I B F Cl ortho-metalation 2-iodoarylacetic acids N-arylation iodination This document was downloaded for personal use only. Unauthorized distribution is strictly prohibited. F Pd(OAc)2 (2 mol%) PhI(OAc)2 (0.75 equiv) I2 (0.75 equiv) Lumiracoxib palladium NH2 Cl CO2H Pd(OAc)2 (2 mol%) PhI(OAc)2 (0.75 equiv) I2 (0.75 equiv) CO2H F (4.0 equiv) CO2H DMF, 60 °C, 12 h 70% (0.5 mmol scale) D Cl I E NH K2CO3 (2.5 equiv) CuI (1.0 equiv) NMP, 100 °C, 12 h 61% (0.5 mmol scale) Cl Cl Diclofenac Some further examples of 2-iodoarylacetic acids generated using the IOAc/PdX2 catalyst system: Br CO2H CO2H CO2H I 82% yield I O 65% yield CO2H I 72% yield 62% yield O CO2H CO2H I F 72% yield CF3 I 75% yield CO2H I I 83% yield AcO PhO Ph CO2H CO2H I 65% yield I 75% yield Significance: Arylacetic acids undergo palladi- Comment: The active precatalyst is PdI2 which um(II)-catalyzed ortho-C–H iodination in the presence of IOAc generated in situ by reaction of PhI(OAc)2 with iodine. The value of the reaction was illustrated by syntheses of the nonsteroidal anti-inflammatory drugs lumiracoxib and diclofenac. A selection of the twenty-three substrates examined shown above illustrates the scope of the reaction. Methoxy-substituted substrates react with IOAc non-selectively in the absence of palladium. The reaction must be shielded from light to prevent radical decomposition of acyl hypoiodite intermediates. can be collected at the end of each reaction and recycled up to five times with only a slight decrease in yield. In most cases 5–15% of the palladium catalyst was required to achieve acceptable yields and conversion. In one case (A → B) just 2% of the catalyst was required. None of the reactions was performed on a substantial scale. For some insight into the possible mechanism of the reaction, see: T.-S. Mei, R. Giri, N. Maugel, J.-Q. Yu Angew. Chem. Int. Ed. 2008, 47, 5215. Philip Kocienski Synfacts 2010, 10, 1103-1103 Publishedonli e:x .x .201 Published online: 22.09.2010 DOI: 10.1055/s-0030-1258072; Reg-No.: K06710SF © Georg Thieme Verlag Stuttgart · New York SYNFACTS Contributors: 2010 © THIEME STUTTGART • NEW YORK 1103