Chapter 7 Laboratory Measurements
advertisement

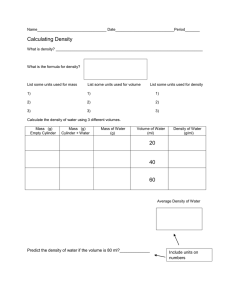
Intro Lab Methods Chapter 7 Laboratory Measurements These notes will only look at those measurements which will be made in Intro Lab Methods. A full discussion of other measurements is available in the text Practical Laboratory Skills. This chapter looks at the following physical properties: • pH • refractive index • density • conductivity pH Measurement of pH is a widely applied technique for monitoring agricultural, industrial and biomedical processes. pH is a measure of the acidity of a sample, a low pH (ie <7) indicates the sample is acidic, a high pH (>7) indicates the sample is basic. pH may be measured using: 1. indicator papers: paper impregnated with coloured dyes, whose colour is sensitive to the pH of the solution to which they are exposed 2. indicator solutions: solutions which are made up from the same coloured dyes which are used for indicator papers. They are added directly to the test material where the coloured form is used to assign a pH value. 3. pH meter: an electronic instrument which gives a direct readout of pH. It uses a glass probe which generates an electrical signal in proportion to the hydrogen ion concentration of the solution in which it is immersed. The pH meter must be calibrated prior to use to ensure the validity of the result. Refractive index The refractive index of a substance is defined as the ratio of light velocity in a vacuum to its velocity in the particular substance. For routine laboratory applications, velocities are not measured. Instead the bending caused by the velocity change between the vacuum and the material is measured. Because the substance always slows down or retards the passage of the light beam, the light beam is bent or refracted and the amount of the refraction or refractive index is measured. In routine laboratory practice, the major factors which affect RI values are: • the material • the temperature • the wavelength of light used • the purity of the sample The standard instrument for measuring RI is the refractometer. 58 | P a g e Intro Lab Methods Conductivity measurements Solutions which contain ions will conduct electricity, just like a metal does. Conductivity is the ease with which a current can be carried by a solution. Conductivity is measured using a special probe attached to a digital or analogue meter. The meter must be calibrated in order for the value obtained to have valid significance Measurement of density The density of any gas, liquid or solid is its mass per unit volume. mass Density = volume Mass is measured with a balance Volume is measured by direct reading of a volume scale on the container or from displacement measurements by immersion in a suitable liquid. The object is immersed in the liquid and the apparent rise in level equals the volume of the object. Problems arise when: • the material is soluble • the void spaces absorb fluid • the material dissolves, swells, expands or shrinks Liquid density measurements Two basic methods are used 1. density bottles (pycnometers): highly precise volumetric vessels which are filled with the liquid in question and weighed. 2. hydrometers: specially weighted floats which are immersed in the liquid. Refractive index can be correlated with density. The producers and users of sugarbased materials make extensive use of density as measured by hydrometry and refractometry to analyse for sugar content. Practical work 7.1 pH measurements The practical tasks provide experience with various detection methods for the determination of pH. Your teacher will demonstrate the use of each method. Whilst working with each consider the accuracy and efficiency of the method. Practical work 7.2 Calibration of pH meter Practical work 7.7 Measurement of density The practical is designed to give the student skills in determining the density of a variety of different types of material, (irregular solids, granular solids, liquids etc) using a variety of techniques (hydrometry, pycnometer etc). 59 | P a g e Intro Lab Methods Density of an irregular solid 1. obtain three objects and record their identity 2. measure and record the mass of the objects 3. partially fill an appropriate measuring cylinder with water and record the initial volume 4. Carefully immerse the object in the measuring cylinder 5. record the new volume 6. complete the worksheet Bulk density of various granular solids 1. obtain three different granular solids 2. record the mass of an appropriately sized dry measuring cylinder 3. add a predetermined volume of sample to the measuring cylinder 4. reweigh the measuring cylinder, record the mass 5. gently tap the measuring cylinder to pack down the granular solid 6. record the compacted volume 7. add a known quantity of water to the measuring cylinder 8. record the new volume in the measuring cylinder 9. calculate the true volume of the granular solid 10. complete the worksheet Density of liquids 1. weigh and record the mass of a clean, dry 25mL measuring cylinder 2. add 25 mL of the liquid sample to the measuring cylinder 3. reweigh the measuring cylinder and record the mass 4. complete the worksheet 5. follow the teacher’s instructions regarding disposal of the organic solvents Density of liquids by hydrometry 1. follow the teachers instructions regarding use of the hydrometer 2. carefully float the appropriate hydrometer in a measuring cylinder of the allocated liquid 3. read the value from the bottom of the meniscus Determination of sugar content by hydrometry and refractometry 1. carefully measure the hydrometer reading of each of the sugar solutions provided, ensuring that the hydrometer is dried before placement in a new solution 2. carefully measure the refractive index of each of the solutions following instructions given by the teacher 3. determine the concentration of sugar in each of the unknowns by plotting i) Hydrometer reading vs sugar content ii) Refractive index vs sugar content 60 | P a g e Intro Lab Methods Student Name: Practical: pH measurements Practical Number: 7.1 Date Performed: Text book References Date Submitted: Procedure: Indicator solutions, test papers and the pH meter are used to measure pH. Different requirements for accuracy and speed will dictate which method is appropriate. Complete the table below by determining the pH of the samples provided, using the methods as shown by your teacher Results: Sample Litmus Red Litmus Blue BTB Phenolphthalein Universal indicator Other? Dip stick pH meter 61 | P a g e Intro Lab Methods Questions: Which method do you consider the most reliable? Which household chemical was the most acidic? Which household chemical was the most alkaline? What information does litmus paper give? 62 | P a g e Intro Lab Methods Student Name: Practical: To determine melting point. Practical Number: 7.3 Date Performed: Date Submitted: Text book References see text book P. 152, 153 Results: Sample First sign of melting Completely liquid Literature value Sample 1: benzoic acid Sample 2: 2 – naphthol Mixture of samples 1 & 2: Sample 3: Possibilities are: Unknown Code Mix of Sample 3 + first suspected compound conclusion Mix of Sample 3 + second suspected compound conclusion Unknown code … was found to be Sample 4: Possibilities are: Unknown Code Mix of Sample 4 + first suspected compound conclusion Mix of Sample 4 + second suspected compound conclusion Unknown code … was found to be Questions: Answer the questions at the end of Practical exercise 7.3 in the textbook. 63 | P a g e Intro Lab Methods Student Name: Practical: To measure the refractive index of liquids. Practical Number: 7.5 Date Performed: Date Submitted: Text book References see text book P. 155 Results: A. Refractive index of a variety of solvents: Substance RI reading Temperature Corr. factor Corr. RI Conclusion Water Trichloromethane Unknown Unknown Unknown B. Refractive index of sugar solutions using sucrose calibration standards: RI reading Temp. Corr. factor Corr. RI 5% 10% 20% Unknown 1 Unknown 2 Unknown 3 C. Refractive index of wine solutions using ethanol calibration standards RI reading Temp. Corr. factor Corr. RI 5% 10% 20% Unknown 1 Unknown 2 Unknown 3 Questions: Answer questions at the end of Practical exercise 7.5 in the text book. You will need to prepare calibration graphs for each set of sugar and wine solutions and obtain the answers for the amount of sugar and ethanol in each of the unknowns. 64 | P a g e Intro Lab Methods Student Name: Practical: To measure the conductivity of various liquids. Practical Number: 7.6 Date Performed: Date Submitted: Text book References see text book P. 156 Results: Conductivity of aqueous materials Solute Concentration Instrument reading Units Water KCl KCl KCl NaCl NaCl NaCl NaCl NaCl HNO3 NaNO3 KNO3 Ca(NO3 ) 2 Mg (NO3 ) 2 Zn (NO3 ) 2 Questions What trends exist for your NaCl solutions? Comment on which cations and anions are better conductors. 65 | P a g e Intro Lab Methods Student Name: Practical: Density Practical Number: 7.7 Date Performed: Text book References Procedure: Results: 1. Density of an irregular solid Sample mass (g) Initial Identity Volume or code (mL) Date Submitted: Final Volume (mL) True Volume (mL) Density (g/mL) Literature Density You need to reference the source of your literature value data here. 2. Bulk density of various granular solids Sample I.D. Empty cylinder mass (g) Sample + Cylinder Mass (g) Mass sample (g) Bulk volume (mL) Settled volume (mL) True volume (mL) Bulk density Settled density True density Summary. Bulk Settled True density Sand densities are Styrene bead densities are Saw dust densities are 66 | P a g e Intro Lab Methods 3. Density of liquids & 4. Density of liquids by hydrometry Sample I.D. Empty cylinder mass (g) Sample + cylinder mass (g) Sample mass (g) Volume sample (mL) Density (g/mL) Hydrometer reading Lit. Density 5. Sugar content by hydrometry Sample identity Hydrometer reading Refractive index 5% sugar 10% sugar 20 % sugar 30% sugar unk 1 unk2 You must plot a calibration graph (manually or by excel) for your RI and hydrometer results and use it to calculate the sugar content of each of your unknowns. Concentration unk 1 = = _________________ % (by hydrometer) _________________ % (by RI) Concentration unk 2 = = _________________ % (by hydrometer) _________________ % (by RI) 67 | P a g e