7 Fourier Transform Techniques

7
Fourier Transform Techniques
7.1
What is a Fourier transform?
A Fourier transform (FT) is not an analytical instrument in its own right; in fact it isn’t even a real object, but rather a mathematical equation!
It is a mathematical procedure developed by a French mathematician by the name of Fourier for converting complex waveforms into a combination of sine waves (see Figure 7.1), which are distinguished by their intensity and frequency.
This may not sound terribly relevant to chemical instrumentation: making the connection is what this chapter is about.
Combination
Wave 1
Wave 2
FIGURE 7.1
Fourier transform conversion of combination waveform to separate waves
To explain how the FT works, it is easiest to use a simple emission spectrum of three lines, which would look like Figure 7.2(a).
This type of spectrum is known as a frequency domain spectrum , since it is a plot of intensity versus frequency/wavelength.
This is the normal spectrum that chemical analysis is based on.
There is another way of expressing this spectrum: in the form of a time domain spectrum , where intensity is a function of time (as in Figure 7.2(b).
This shows the oscillation of intensity over time, and is not a smooth sine wave, because it is the combination of three different waveforms – the three emission wavelengths.
(b)
(a)
I
1
sin f
1 t
+
I
2
sin f
2 t
Frequency Time
FIGURE 7.2
Two emission lines as a (a) frequency domain and (b) time domain spectrum
7.
Fourier Transform Techniques
The time domain spectrum is of no practical use for chemical analysis, because it is the individual lines that we need.
However, the Fourier transform calculation processes a time domain spectrum and delivers the information needed for a frequency domain spectrum: frequency/wavelength and intensity of the individual waveforms (i.e.
emission lines).
Time Domain Spectrum FT Frequency Domain Spectrum
Is there an advantage of measuring the time domain spectrum?
The time variations in intensity can be measured without the need to scan through individual wavelengths, because the combined waveform contains all the necessary information for the FT to
produce a frequency domain spectrum.
But there’s a problem!
To measure the time domain spectrum, we need a detector capable of responding to the variations in intensity of electromagnetic radiation.
If we choose a visible wavelength (e.g.
500 nm) as an example, then it has a frequency of 6 x 10
14
Hz, or 600,000,000,000,000 oscillations per second!
This
means a detector would have to have a mind ‐ boggling response time to see these oscillations.
C LASS E XERCISE 7.1
Most detectors have a response time of 100 milliseconds.
This means they only see an average of what occurs each 100 ms.
(a) How many oscillations will occur while the detector responds?
(b) What will be the output from the detector?
So we are stuck with a good idea and no way to make it work!
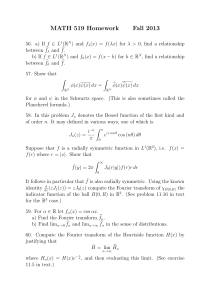
7.2
How to make the Fourier transform technique workable
In the late 1800s two scientists, Michelson and Morley, built a device which was intended to prove that light moved at different speeds in different directions.
The purpose of this was to show that a substance known as an “ether” existed, through which the waveform of light was transmitted.
The device was known as an interferometer , and is based on the idea of constructive and destructive interference.
Waveforms when they overlap “combine” and the resulting intensity depends on the level of overlap.
As it turned out, Michelson and Morley found that they could not prove what they hoped (because the ether didn’t exist and light moves the same speed in all directions).
The interferometer was consigned to the museum of scientific curiosity, until some very bright person realised that the
Fourier transform and the interferometer could be combined to make a working spectrometer.
AIT 7.2
7.
Fourier Transform Techniques
How does an interferometer work?
Figure 7.3
shows the basic components.
Fixed Mirror
Interferometer
Moveable Mirror
Radiation
Source
beam splitter
Detector
FIGURE 7.3
Schematic diagram of an interferometer
How does the interferometer work?
Radiation from the source hits a beam splitter and 50% is reflected at right angles towards the fixed mirror, and 50% passes through to the moveable mirror.
The two beams bounce from the mirrors and are recombined at the beam splitter and are diverted to the detector.
So far, so good.
If the distance that the two beams travel after splitting is exactly the same then they will recombine perfectly in phase, meaning that the overall intensity is not decreased.
If the second mirror is moved, the paths will be of different lengths, and the recombined beams will be out of phase, causing interference that reduces the intensity of the beam.
Thus, the detector sees a reduction in intensity.
As the mirror moves steadily along a path of a few centimetres, the intensity at the detector varies due to the varying interference, producing an interferogram .
Now comes the miracle!
The interferogram is an exact replica of the waveform of the radiation form the source, with a frequency that is directly proportional to the real frequency of the radiation .
It does not matter what shape the incoming waveform is, the interferogram will replicate it.
Now, we have the means for producing a waveform that can be detected, and then processed by the Fourier transform calculation.
But we need a way of determining what the relationship between the source and interferogram frequencies is.
Well, a simple equation exists that links the two by using a source of exactly known wavelength: a laser.
So at the moment we have a way of determining the spectrum of a source.
But in the case of an absorption instrument such as infrared, we want to measure the spectrum after it has been absorbed by the sample.
Thus, the sample cell must be located into the configuration shown in Figure 7.3.
In all reference books, the sample cell is shown placed between the interferometer and the detector .
(It must be said that the author of these notes cannot see how this can work, and that the logical position is between the source and the interferometer.) The Fourier transform technique, together with the interferometer, has been used in spectroscopic instruments such as infrared, NMR, Raman and MS (though how the latter works without radiation being involved is a bit of a mystery!).
AIT 7.3
7.
Fourier Transform Techniques
With real spectra, the combination of wavelengths is exceedingly complex because we are dealing with polychromatic radiation.
While the Fourier transform can process any waveform into any number of sine waves, computer technology was not sufficient until about 20 years ago to make it viable.
7.3
Advantages of Fourier transform instruments
A Fourier transform ‐ based spectrometer has all the advantages of a multi ‐ channel instrument, without the extra cost of multiple detectors, and also has extra ones of its own.
These advantages are:
speed – the only moving part in the instrument is the mirror, and its travel only occupies a few seconds; spectra can be recorded in milliseconds with reduced mirror travel (this is expanded on below)
wavelength accuracy – the Fourier transform and the laser ‐ calibrated interferometer achieve spectral accuracy undreamt of in conventional instruments (eg 0.01
cm ‐ 1 )
greater sensitivity – because there are fewer optics inside the instrument, compared to a monochromator ‐ based one, more radiation is passing through the sample, allowing improved sensitivity
better quantitative performance – the wavelength accuracy and sensitivity combine to allow far better quantitative accuracy, with absorbances of greater 2 still giving linear performance
The speed advantage of FT instrument leads to two other advantages: noise reduction and time ‐ resolved spectra.
Background noise is a problem for low concentration samples, and obscures the peak.
This is the known as the signal ‐ to ‐ noise ‐ ratio (S/N), and where the signal is low, the S/N ratio will be low as well.
Because the FT instrument is so fast, we can record multiple spectra in less than the time normally taken for a monochromator scan.
The computer then averages these spectra, and the noise will
“disappear” because at a given frequency, in some spectra it will be high, and in others it will be low, the average being somewhere in the middle.
The peak will also be the same intensity, so it will remain unchanged by the averaging (see Figure 7.4).
FIGURE 7.4
Multiple spectra (pale lines) and average spectrum (dark heavy line)
AIT
7.4
7.
Fourier Transform Techniques
All of the advantages so far described are improvements on the older monochromator ‐ based instruments.
There is one advantage of FT instruments that older instruments cannot do: record spectra so quickly that you can see a chemical reaction actually occurring.
Figure 7.5
shows an example of this: the sequence of spectra are recorded at 400 microsecond intervals .
The FT processing occurs afterwards.
FIGURE 7.5
Time ‐ resolved infrared spectra (spectra of acetone undergoing a photochemical reaction, recorded at 400
s intervals, unknown source)
What You Need To Be Able To Do
define important terminology
explain what the Fourier transform does
explain how an interferometer works
draw a schematic diagram of a FT instrument
explain why an interferometer is required
list instruments using Fourier transform techniques
outline advantages of FT instruments
AIT 7.5
7.
Fourier Transform Techniques
Revision Questions
1.
Draw a diagram showing the difference between a time ‐ domain and frequency ‐ domain spectrum for monochromatic radiation.
2.
Why is the time ‐ domain spectrum superior?
Why can’t it be measured for normal radiation?
3.
What does the Fourier transform do in terms of the two types of domain spectra?
4.
What is the name of the special component in a FT instrument?
What does it replace in conventional instruments?
5.
Give three advantages of FT instruments over conventional equivalents?
6.
Give one application of FT instruments that is not possible at all with a conventional equivalent.
Answers on the next page.
AIT 7.6
7.
Fourier Transform Techniques
Answers to revision questions
Where the answer can be found directly in your notes, a reference to them will be provided.
1.
Figure 7.2
2.
No need to scan.
Detectors can’t respond quickly enough.
3.
Converts from time to frequency domain.
4.
Interferometer.
Monochromator.
5.
Section 7.3
6.
Figure 7.5
and associated text
AIT 7.7