4 I S
advertisement
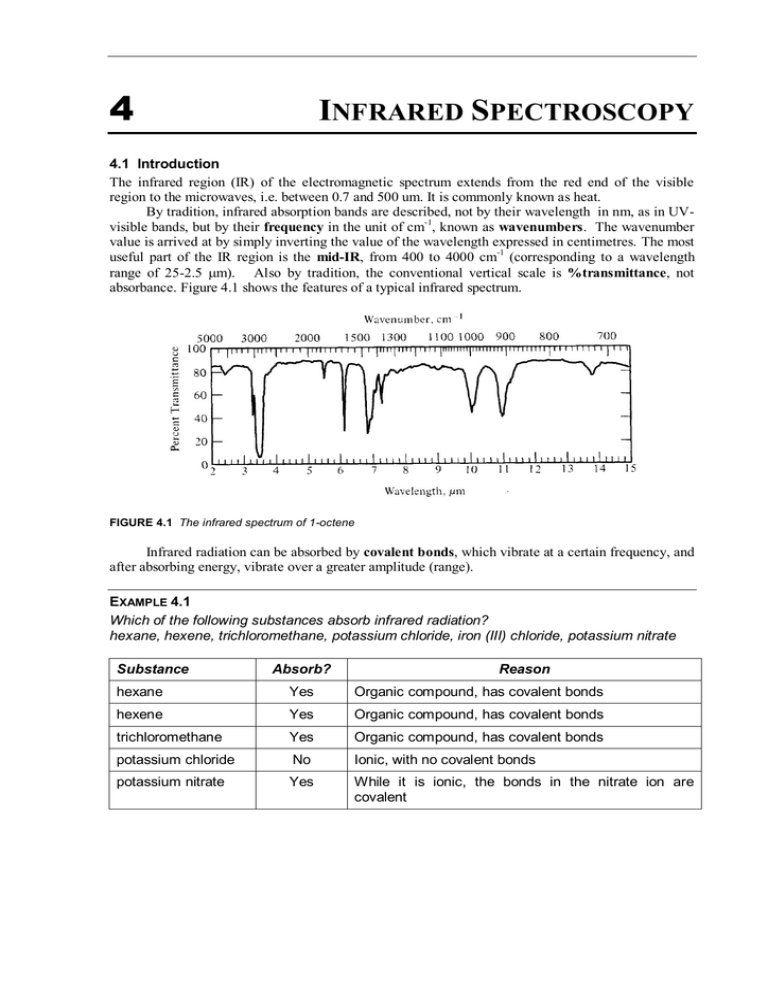
4 INFRARED SPECTROSCOPY 4.1 Introduction The infrared region (IR) of the electromagnetic spectrum extends from the red end of the visible region to the microwaves, i.e. between 0.7 and 500 um. It is commonly known as heat. By tradition, infrared absorption bands are described, not by their wavelength in nm, as in UVvisible bands, but by their frequency in the unit of cm-1, known as wavenumbers. The wavenumber value is arrived at by simply inverting the value of the wavelength expressed in centimetres. The most useful part of the IR region is the mid-IR, from 400 to 4000 cm-1 (corresponding to a wavelength range of 25-2.5 m). Also by tradition, the conventional vertical scale is %transmittance, not absorbance. Figure 4.1 shows the features of a typical infrared spectrum. FIGURE 4.1 The infrared spectrum of 1-octene Infrared radiation can be absorbed by covalent bonds, which vibrate at a certain frequency, and after absorbing energy, vibrate over a greater amplitude (range). EXAMPLE 4.1 Which of the following substances absorb infrared radiation? hexane, hexene, trichloromethane, potassium chloride, iron (III) chloride, potassium nitrate Substance Absorb? Reason hexane Yes Organic compound, has covalent bonds hexene Yes Organic compound, has covalent bonds trichloromethane Yes Organic compound, has covalent bonds potassium chloride No Ionic, with no covalent bonds potassium nitrate Yes While it is ionic, the bonds in the nitrate ion are covalent 4. Infrared Spectroscopy CLASS EXERCISE 4.1 State whether the following compounds would absorb photons from the infrared region: sodium chloride sodium sulfate water benzene For all but the simplest molecules, there will be a large number of vibrations capable of absorbing IR radiation across a range of energies. This produces a complex spectrum, but most importantly, a unique spectrum: a compound's IR spectrum is a "fingerprint". It is this feature that is normally exploited by analysts using IR spectroscopy: qualitative identification, rather than quantitative determination of concentration. 4.2 Instrumentation The majority of infrared absorption instruments are scanning spectrophotometers, because qualitative identification from the spectrum is the main use of the technique. There are specialised non-scanning instruments for air pollution analysis of various gases, such as hydrocarbons, but they are beyond the scope of this subject. Until the last 15 years or so, infrared spectrophotometers were all double-beam instruments, the configuration of which is shown below in Figure 4.2. Since then, a new generation of instrument, known as a Fourier transform IR spectrophotometer, has become popular for a number of reasons, particularly accuracy and speed. Reference cell Radiation source Beam splitter Sample cell Monochromator Detector Readout FIGURE 4.2 A typical double-beam IR instrument The function of the components in an IR instrument are the same for equivalent components in any other type of spectrophotometer, but the actual type and design will be different because of the different region of the spectrum being measured. 4.3 Sample Preparation The major difference in practical terms between infrared and UV-visible spectroscopy is in the requirements of sample preparation. The plastic and quartz materials used in UV-visible cells, and solvents, such as water, ethanol and hexane, are useless in IR spectroscopy. Lower sensitivity of the IR process means that the mg/L concentrations used in UV-visible work are far too dilute. Cell Materials The most common material which is IR-transparent is NaCl, and plates of crystalline salt are used as sample cells. Two distinctly different cell types are used for liquid samples. One features two NaCl plates held together by metal frames, with a narrow sample volume in the centre. These are most Sci Inst Analysis (Spectro/Chrom) 4.2 4. Infrared Spectroscopy commonly of a 0.1 mm path length, though other path lengths, e.g. 0.025 and 0.05 mm, are available. The are known as fixed-path length or sandwich cells, and are used for solutions. The other cell type relies simply on surface tension of the liquid between two loose NaCl plates to hold them together. They are suitable for pure liquids, but not for solutions, because there is no way of compensating for solvent absorption. (a) (b) metal frame NaCl plates sample inlets solution space FIGURE 4.3 Infrared cells (a) fixed-path length (b) loose plates The obvious problem with these cells is that they must be kept away from water as much as possible. Even atmospheric humidity is sufficient over time to fog the cells, therefore requiring repolishing. You also must not touch the surfaces of the cells because of the moisture and oils on your fingers, which will degrade the materials and the spectra. Solid materials, which are not soluble in suitable solvents or have certain undesirable characteristics in solution, can be ground into powder and their spectrum recorded in either of two ways: as a KBr disc, where the material is dispersed in finely ground KBr, and compressed at very high pressures (up to 20 tonnes) into a thin disc. as a mull, where the solid is suspended in a viscous liquid, such as mineral oils (e.g. Nujol), or The former method – the KBr disc – is the better one, though it is a skill needing practice to make them properly. It is superior to a mull and in fact to a solution, because there are no interferences. There is no solvent to cause peaks and require correction and KBr, like NaCl, has no covalent bonds and so no peaks. Solvents Solutions require concentrations of around 1-10% w/v, in the solvent trichloromethane (chloroform, CHCl3). Tetrachloromethane (carbon tetrachloride, CCl 4) is a better IR solvent, but is carcinogenic, so is no longer used. Neither solvent are not transparent to infrared radiation (i.e. they absorb some frequencies of radiation), but have sufficiently simple spectra that they can be corrected for by a reference of the pure solvent. The fixed pathlength cells must be used where a solution is involved, so that an accurate correction of the solvent absorption can be made. 4.4 Analysis using IR absorption Quantitative Analysis The infrared spectrum of even a simple compound is complex with many peaks. Therefore, mixtures have very complex spectra, making the technique unsuitable for quantitative work in general. It is possible to do so using Beer's Law, but it is necessary to remember that conventional infrared instruments produce a transmittance spectrum which therefore requires conversion to absorbance. However, the major problem is that it is necessary to find a baseline against which the transmittance of the analyte can be measured. This baseline should be horizontal, and this is extremely difficult to find in the complex array of peaks in most spectra. Furthermore, if the sample is a mixture of more than one compound, the spectrum becomes very difficult to interpret, and standards are difficult to prepare. Sci Inst Analysis (Spectro/Chrom) 4.3 4. Infrared Spectroscopy Qualitative Analysis The infrared (IR) spectrum of a pure substance is unique, in the same way that human fingerprints are. At first glance, IR spectra of molecules are highly complex, for reasons discussed previously. Nevertheless, very small changes in molecular structure lead to clear differences in the infrared spectra, as is shown in Figure 4.4. Two different types of information can be obtained from an infrared spectrum: the presence or absence of different atomic groupings (functional groups), in the high frequency (> 1500 cm-1) part of the spectrum a unique fingerprint of the compound, in the low frequency (< 1500 cm-1) part of the spectrum EXAMPLE 4.2 Identify the functional group and fingerprint regions in the spectrum in Figure 4.1. FIGURE4.4 Functional group region Fingerprint region CLASS EXERCISE 4.2 Identify the functional group and fingerprint regions in the spectrum in Figure 4.4. Identification of an unknown compound is possible, since sets of standard IR spectra are commonly available. These spectra, numbering many thousands, are generally grouped together on a chemical basis, e.g. all the spectra of alkanones are together. This reduces the tedium of searching through vast numbers of spectra if the chemical nature of the substance is known. When trying to identify a compound by looking through books, you need to know something about the functional groups present. This is done either by chemical means or by using certain characteristic absorption peaks (bands) which occur in the functional group region, a number of which are shown in Table 4.1. Figure 4.6 shows the infrared spectra of three isomeric compounds, each possessing different functional groups. TABLE 4.1 Characteristic absorption frequencies of certain functional groups Functional Group Frequency range (cm-1) Alkane (C-C-H) * 2800-2950 Alkene (C=C-H) 3050, 1620 Alkanol (OH) 3400-3500 Alkanone, alkanal (C=O) 1680-1720 Benzene ring 3040, 1600, 1500, 1450 * the substance does not need to be simply an alkane to have peaks here, but rather to have C-C-H bonds Sci Inst Analysis (Spectro/Chrom) 4.4 4. Infrared Spectroscopy EXAMPLE 4.3 Identify the functional groups in 1-octene and find their peaks in Figure 4.1. 1-octene has the structure CH2=CH-(CH2)5CH3 Its functional groups are alkane (C-C-H) and alkene C=C-H. alkene 3050 alkane 2800-2950 alkene 1620 CLASS EXERCISE 4.3 Identify the functional groups in the compounds and find their peaks in Figures 4.5 and 4.6. Now use its fingerprint region and don’t worry any more about the functional group region. Look for a “pattern” in your spectrum (eg three peaks in descending order, or one big peak by itself). Now try to find that pattern in the pages and pages of spectra. While many compounds with the same functional groups will have similar spectra in the functional group region, they will all have unique fingerprint regions, and therefore, identification is made by finding an essentially perfect match between the fingerprint region of the unknown and the standard. Your spectrum must match the reference spectrum perfectly: no extra peaks no major peaks missing consistency in the relative intensities of each peak Different sample preparation methods (e.g. KBr disc and CHCl 3 solution) will give rise to small variations in frequency (±10 cm-1) and intensity, but the changes will not make much difference to the appearance of the spectrum. Computer programs are now available, in which the frequencies of the standard spectra have been inputted into a database. The frequencies of 4-8 of the most intense bands in the unknown spectrum are entered by the operator, and the computer searches for any matches. It is then a matter of checking the actual spectra for the exact match. Sci Inst Analysis (Spectro/Chrom) 4.5 4. Infrared Spectroscopy (a) (b) (c) FIGURE 4.5 Spectra of (a) 1,2- (b) 1,3- and (c) 1,4-dimethylbenzene Sci Inst Analysis (Spectro/Chrom) 4.6 4. Infrared Spectroscopy (a) (b) (c) FIGURE 4.6 Spectra of (a) cyclohexanol (b) 2-hexanone and (c) butoxyethene Sci Inst Analysis (Spectro/Chrom) 4.7 4. Infrared Spectroscopy What You Need To Be Able To Do give the unit of frequency most commonly used in infrared spectroscopy state what features of a molecule absorb photons of IR radiation identify whether a substance would absorb IR radiation draw a diagram showing the components of typical scanning spectrophotometers describe the cells and solvents used in IR spectrophotometry describe and compare the methods for recording the spectra of solid and liquid samples explain the principles of qualitative analysis using IR spectroscopy distinguish between the functional group and fingerprint regions Terms And Definitions Match the term with the definition. 1. 3. wavenumber functional group region 2. 4. fingerprint region mull A. B. C. D. a suspension of solid material in an oil for the purpose of recording a spectrum of the solid the region in an IR spectrum for identifying the presence of common atomic groupings the frequency unit in IR spectra the region in an IR spectrum that is unique for a specific compound Review Questions 1. What class of compounds do NOT give an infrared absorption spectrum? Explain your answer. 2. Why must solution spectra be run using fixed pathlength cells? 3. Why are there no solvents available that absorb NO infrared radiation at all? 4. Describe TWO methods of producing an infrared spectrum of a solid substance. 5. Explain how an infrared spectrum can be used to definitely identify an organic compound. 6. What are the (i) functional group and (ii) fingerprint regions of the infrared spectrum? What information does each provide? 7. A chemical process produces cyclohexanone by the oxidation of cyclohexanol. Describe a monitoring system using infrared spectroscopy for the reaction. 8. Identify which of the following infrared spectra would be characteristic of pentane, 2-pentene, 1-pentanol, 2-pentanone, and pentylbenzene. Sci Inst Analysis (Spectro/Chrom) 4.8 4. Infrared Spectroscopy Sci Inst Analysis (Spectro/Chrom) 4.9