BASIC CONCEPTS
advertisement

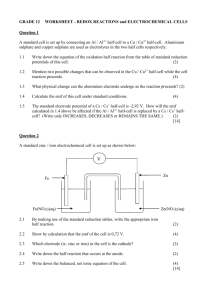
1 BASIC CONCEPTS 1.1 Electroanalytical Techniques The ability of chemical species in solution to conduct electricity, and to undergo electron-transfer reactions can be exploited analytically, both for identification purposes and, more commonly, quantitative determinations. The three basic electrical measurements, voltage (or potential), current and resistance (or its inverse, conductivity) are all employed in instrumental analytical techniques. In general, one of these is measured while the other two are maintained at a constant level. In this module, we shall look at all the common electroanalytical techniques. TABLE 1.1 Summary of electroanalytical techniques Technique Measured Parameter Potentiometric titrations * Change in potential during titration due to change in concentration of certain species Direct potentiometry (ionselective electrodes) * Solution potential compared to that of specific ion standards Conductivity * Solution resistance as a measure of dissolved ions Conductometric titrations * Change in solution resistance during titration due to change in concentration of ionic species Amperometric titrations Change in current flow during titration due to change in concentration of ionic species Coulometry Charge (current x time) required to cause complete reaction with analyte Electrogravimetry Analyte electrically precipitated and weighed Voltammetry Current measured as a function of applied potential * covered in this subject 1.2 Electrochemical Cells When a species is oxidised, it transfers electrons to the other species involved in the reaction. This, at a molecular level, is akin to a flow of electrical current. After all, what is electrical current but a flow of electrons from one place to another. In a redox reaction in a beaker, the electrons go directly from molecule to another. If, however, we could separate the two processes - reduction and oxidation - so that electrons had to flow between one beaker and another via a wire, then we have the capacity to generate a measurable (and useful) electrical current. In a modified form, all our batteries (in your calculator, car etc) fit this description: a chemical reaction producing electrical current. In the laboratory, how would such a setup work? Figure 1.1 shows a typical apparatus, using copper, zinc and solutions of their ions. For the moment, ignore what the salt bridge might be. What will happen in this system? The system is known as an electrochemical cell, where the redox processes are separated electrically into half-cells. Two possible half-reactions (or their reverse) can occur: Cu2+ + 2e ↔ Cu Zn2+ + 2e ↔ Zn It should be clear by now that both can’t occur in the reduction form, since this would mean that electrons were appearing from nowhere. So, one metal will be oxidised, providing the electrons for the ions of the other metal to be reduced. 1. Basic Concepts connecting wire Zn Cu Salt bridge Zn 2+ Cu2+ FIGURE 1.1 A typical electrochemical cell As it turns out, the zinc is oxidised, and the copper (II) ions are reduced. CLASS EXERCISE 1.1 (a) Write the two half-equations in the correct form that shows which is the reduction half and which is the oxidation half. (b) On Figure 1.1, show how the electrons travel. (c) What will happen over time to: (i) the zinc electrode (ii) the zinc solution (iii) the copper electrode (iv) the copper solution (d) Given this is an equilibrium process, what do you think will happen eventually? SIA (Electro) 1.2 1. Basic Concepts The zinc and copper serve two purposes: in the case of the zinc, as source of electrons, and for the copper, as a receiver of the electrons. In each case, they are acting to transport (conduct) the electrons from one half-cell to the other, and are known as electrodes. The zinc electrode is an essential part of the reaction, because the zinc atoms are being oxidised. However, the copper electrode is not part of the reaction, and could be replaced by any other material that did not react, and was conductive, eg platinum, gold, graphite. In all electrochemical measurements, there must be TWO electrodes. What is the salt bridge? It is a non-reactive conductive material, possibly as simple as a piece of paper soaked in a salt solution, or a frit, which is a porous material (eg glass) which allows movement of liquid through it. What is it doing? It completes the electrical circuit. Why is it necessary? Think about what happens each time a copper ion is reduced and a zinc atom is oxidised. The copper beaker loses a 2+ ion, and the zinc beaker gains a 2+ ion. If this continued (or was even possible), the copper beaker would become negatively charged and the zinc beaker positively charged. This is impossible. What needs to happen to balance up charge is the unreactive ions (anions in this case) have to migrate from one beaker to another to keep the charge in each balanced. Thus, the nitrate ions in the copper beaker pass through the salt bridge into the zinc beaker. 1.3 Voltage Measurements In the cell in Figure 1.1 above, you were told that it is the zinc that is oxidised, and the copper ions that are reduced. Why not the other way round? Electrons flow in an electrochemical cell because of a voltage (or potential) difference between one electrode and another. Electrons flow from the electrode with the greater ability to lose them. It is like water in a bucket half way up a hill. If you tip the bucket over, the water runs downhill, not uphill, because of the laws of gravity. Some species have a greater need to lose (or gain) electrons than others. Zinc is more stable as the 2+ ion than the metal, so if it is put into contact with a species that wants to accept the electrons (eg copper ions), then a reaction will occur. However, if the zinc metal is put into contact with a species that doesn’t want the electrons (eg sodium ions), then nothing will happen. If the zinc ions are put into contact with something that has an even stronger need to lose electrons (eg aluminium), then the zinc ions will be reduced. The potential (or ability) of a half-cell to lose or gain electrons can be measured in volts by a voltmeter, but not by itself. There must always be a something there to accept/lose the electrons, ie a second half-cell, which will complete the circuit. This half-cell has its own potential, so any measurements of potential (voltage) can only be relative between the two half-cells. If this causes you a problem, imagine yourself standing on a wall between two bodies of water (the two half-cells). You have a measuring stick (a voltmeter), which can reach the surface of each body of water. You want to measure the depth of one of the bodies of water, but your measuring stick isn’t long enough. However, you can measure the difference in their depths (potential difference). Figure 1.2 illustrates this idea. The voltage for a half-cell is dependent on the species involved, but also their concentrations, so it is possible to make use of voltage measurements to analyse solutions. However, if you want to measure the voltage of a solution you are interested in, you have to connect it to another half-cell, meaning that the value can only be relative to that other half-cell, which you are not interested in! And if someone else measures the solution you are interested in, connected to a different half-cell, they get a different answer. Which is definitely not very useful! SIA (Electro) 1.3 1. Basic Concepts height difference FIGURE 1.2 The ‘depth of water’ analogy for potential difference So how do we get round this problem? By using a standard half-cell as a comparison point – known as a reference cell or electrode. It doesn’t allow us to measure the voltage of our solution by itself, but at least we get a consistent value. This idea has been taken one step further: one particular reference electrode, called the standard hydrogen electrode, has been given the value of 0.00 V. All other cell voltages are measured relative to it. Table 1.2 lists the standard reduction potentials of a number of half-reactions. You can see the half-equation in the middle of the table at 0.00 V. The reduction of hydrogen ions to hydrogen gas defines the standard hydrogen electrode. Some points about standard reduction potentials 1. The term “standard” refers to 25°C and 1 M solution concentration. 2. The half-equations are written in reduction form for consistency sake. They are all reversible. 3. The more positive the voltage, the more likely the half-reaction is to occur; the more negative, the less likely. 4. To obtain the potential for the process as an oxidation, simply change the sign of the voltage, eg the standard potential for the oxidation of iron (II) to iron (III) is –0.77V. CLASS EXERCISE 1.2 Using Table 1.2, identify (a) which species is most readily reduced (b) which species is most readily oxidised (c) which metallic element is most easily oxidised (d) which metallic element is least easily oxidised SIA (Electro) 1.4 1. Basic Concepts TABLE 1.2 Some standard reduction potentials Half reaction - +2.87 F2 + 2e ↔ 2F + +1.78 3+ +1.61 H2O2 + 2e + 2H ↔ 2H2O 4+ Ce + e ↔ Ce - + MnO4 + 8H + 5e ↔ Mn 2+ + 4H2O Au3+ + 3e ↔ Au Cr2O7 2- + 14H + 6e ↔ 2Cr 3+ + 7H2O + + e ↔ Fe 2+ - 2+ SO4 +0.54 Sn 4+ +0.34 + 2e ↔ Cu 2- + + 4H + 2e ↔ SO2 + 2H2O + 2e ↔ Sn +1.23 +0.77 I2 + 2e ↔ 2I Cu +1.33 +0.80 Ag + e ↔ Ag 3+ +1.51 +1.50 + O2 + 2H2O + 2e ↔ 4OH- Fe Potential Eo (V) 2+ +0.17 +0.15 Pb2+ + 2e ↔ Pb +0.03 2H+ + 2e ↔ H2 0.00 S4O6 2- + 2e ↔ 2S2O3 2- Cd2+ + 2e ↔ Cd -0.08 -0.40 2+ -0.44 2+ -0.76 Fe + 2e ↔ Fe Zn + 2e ↔ Zn + Li + e ↔ Li -3.04 Calculating the voltage of electrochemical cells While not particularly useful for chemical analysis, the expected potential for a full electrochemical cell can be calculated simply as the difference between the potentials of the two half-cells. EXAMPLE 1.1 Calculate the potential for the copper and zinc half-cells. E = ECu – EZn = 0.34 – (-0.76) = 1.1 V What if you subtract them the other way round? You get the same number but the opposite sign. The positive or negative sign does mean something, but is not really important for this subject. SIA (Electro) 1.5 1. Basic Concepts CLASS EXERCISE 1.3 Calculate the value of the cell potential for the following cells: (a) silver ions and cadmium ions (b) permanganate and iron (II)/iron (III) Voltage and concentration Electrode potentials are dependent on concentration, which means their measurement can be used to tell something about the composition of a solution. Voltage measurements are among the most important methods used in analytical instrumentation to determine the composition of solutions. The Nernst equation (Eqn 1.1) relates the solution concentration to measured voltage, assuming a temperature 25°C. There is a more complicated version of the equation for different temperatures. E = Eo − 0.059 log10 K n Eqn 1.1 where E is the half-cell potential, E° the standard potential, n the number of electrons in the half-equation, and K the value of the equilibrium constant. EXAMPLE 1.2 Calculate the reduction potential for a 0.1 M Cu2+ solution. 2+ The half-reaction is Cu + 2e ↔ Cu, which has a standard reduction potential of +0.34V. The equilibrium constant for the half-reaction is: K = 1 Products over reactants; solid species count as 1. [Cu2 + ] so the value for K is 10. Substituting these values into Eqn 1.1 gives: E = 0.34 − SIA (Electro) 0.059 log (10) = 0.31 V 2 1.6 1. Basic Concepts CLASS EXERCISE 1.4 Calculate the reduction potential for 0.025 M copper (II) solution So, if we can make a measurement of voltage that can be linked to concentration, then this seems a powerful, and relatively tool for analysing solutions. EXAMPLE 1.3 The voltage of a copper (II) solution (measured relative to the standard hydrogen electrode) is 0.29 V. Calculate the concentration of Cu2+. 0.29 = 0.34 − 1 0.0592 log [Cu2 + ] 2 You do not have to be able to do this calculation, so shortcutting to the answer. [Cu2+] = 0.020 M So, from Example 1.3, we appear to have a method for determining the concentration of a species by a single voltage measurement. Unfortunately, this is not the case. There are a number of factors that mean that the single voltage measurement cannot be directly linked to concentration: • the Nernst equation truly relates activity not molarity to voltage – activity is a theoretical measure of concentration, which takes into account the differing abilities of species to react depending on their concentration, but more importantly, the concentration of species in solution with them; as an analogy, think about walking down Pitt Street in Sydney when it is empty first thing in the morning, compared to when it is full of other people at midday; Table 1.3 shows the effect of on the measured voltage of a zinc solution TABLE 1.3 Effect of activity on cell potential Molarity Zn2+ Bkgd Conc. Calculated E Measured E 5 x 10-4 2 x 10-3 0.608 0.611 2 x 10 -3 8 x 10 -3 0.573 0.583 1 x 10 -2 4 x 10 -2 0.531 0.553 2 x 10-2 8 x 10-2 0.513 0.542 -2 -1 0.490 0.529 5 x 10 • 2 x 10 measurement errors associated with the electrodes – these are not human errors or errors of calibration, but small voltages set up by the contact between electrode and solutions – they are known as junction potentials, and cannot be measured SIA (Electro) 1.7 1. Basic Concepts What does this mean for the use of voltages for analytical measurements. Obviously, there must be some way around these problems, otherwise you wouldn’t be doing this subject! Two approaches are used: • voltage changes during titrations can show clearly the endpoint of the titration, and importantly, we are not using the actual voltage value for anything important, only the change from one titration volume to the next; Chapter 2 looks at potentiometric titrations • use of calibration standards, so that activity and junction potential problems are compensated for; Chapter 3 examines the use of ion-selective electrodes Measuring cell voltages When we measure a solution voltage, we need the following: • one electrode that will respond to the species we are interested in – known as the indicator electrode; it could be the metal in the half-equation (eg silver), or an inert metal in reactions where no metal exists (eg platinum for permanganate) or a special electrode designed to respond to a particular species (eg a pH electrode for hydrogen ions) • a second electrode which does not respond to the solution at all – known as a reference electrode; the standard hydrogen electrode mentioned earlier is not convenient (it requires hazardous hydrogen gas), so others have been designed; the most common is known as the saturated calomel electrode (SCE); it has a fixed potential of 0.244 V at 25°C; Figure 1.3 shows the internal construction of a SCE, AND Table 1.4 outlines the functions of each component • a voltmeter, which the electrodes plug into, and the voltage difference between them is measured; voltmeters actually prevent any chemical reaction occurring because of their design; this means that the solution does not change as a consequence of the measurement Pt wire filling holes for electrolytes saturated KCl solution Hg/Hg2Cl2 paste KNO3 solution porous frits FIGURE 1.3 Construction of a typical saturated calomel electrode SIA (Electro) 1.8 1. Basic Concepts TABLE 1.4 Functions of components of the SCE Component Function Pt wire to provide a conductive connection from the electrode Hg/Hg2Cl2 paste the active components of the half-reaction that drives the SCE; mercury (I) chloride is called calomel KNO3 solution a conductive but inert solution to prevent leakage of chloride ions into sample solutions filling holes to top up the KCl and KNO3 when they run out saturated KCl solution also involved in the half-reaction porous frits to provide electrical contact between the various parts of the electrode and the outside solution What You Need To Be Able To Do • • • • • • • list important electroanalytical techniques draw a typical electrochemical cell explain what is happening in the cell calculate cell potentials calculate the effect of concentration on cell potentials using the Nernst equation explain why the Nernst equation cannot be used directly to calculate concentration list and describe the function of the equipment required for a voltage measurements SIA (Electro) 1.9