N2. ANALYSIS OF CONCENTRATED ACID
advertisement

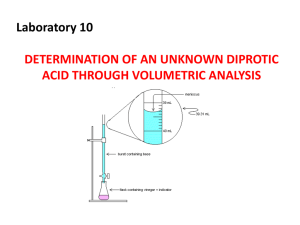
N2. ANALYSIS OF CONCENTRATED ACID PURPOSE: To design and carry out a procedure for the determination of the molarity of a concentrated laboratory acid by titration with standardised 0.1M NaOH PREWORK: This is a group-work exercise and is to be completed and submitted prior to commencement of the experiment You will be assigned one of the concentrated acids below. Write out fully the steps required to determine the molarity of the acid you are assigned. The molarities are as follows: Hydrochloric (HCl) 11.3 M Sulfuric (H2SO4) 18 M Nitric (HNO3) 16 M Your procedure must include: • Dilution steps (note that you should not use volumetric flasks greater than 250 mL or pipettes less than 5 mL). • Aliquot volume and indicator to be used in the titration of the diluted acid • A method for determining the density of the concentrated acid CALCULATIONS 1. Write a balanced equation for the reaction 2. Calculate the moles of NaOH used in the titration and therefore determine the moles of acid involved in the titration 3. Calculate the molarity of the diluted acid 4. Calculate the molarity of the concentrated acid 5. Convert the molarity of the concentrated acid to %w/w (g/100g) of the acid QUESTIONS 1. Compare your answer for molarity to the accepted value. Account for any differences 2. Why is it desirable to have endpoint volumes of a titration between 15 and 30 mL of titrant? 3. What is serial dilution? What are its advantages and disadvantages?