Doonesbury, 5 December 2005
advertisement

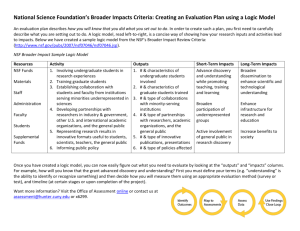
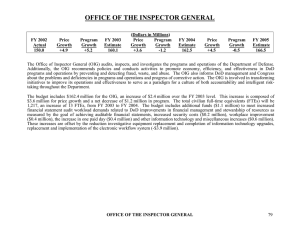
Doonesbury, 5 December 2005 An Investigator Calls What you should know before NSF OIG comes knocking Michigan Technological University 12 February 2008 Scott J. Moore, Ph.D., J.D. Investigative Scientist Office of Inspector General (OIG) Almost every federal agency/entity has one Independent office for oversight Promote economy, efficiency, and effectiveness… Prevent and detect fraud, waste, and abuse… …in agency programs and operations NSF OIG 38 audit staff, 22 investigative staff Investigations staff includes: Ph.D. scientists Accountants (CPA) Special Agents Attorneys Overview What does NSF OIG investigate? Research Misconduct (RM) Regulatory Violations Civil and Criminal Misconduct Where do you fit in? Investigation Committee Member? Witness? Subject? What happens at the end of the investigation? What does NSF OIG investigate? The simple answer Allegations of: Lying Cheating Stealing NSF OIG Inquiries/Investigations Administrative Research Misconduct (RM) Conflict of interests (COI) Other regulatory violations and grant administration issues Civil/Criminal Misconduct False statements and False claims Embezzlement and other financial crimes Mail fraud and Wire fraud More and more frequently we are encountering “hybrid” cases How does OIG know what to investigate? Allegations from Program officers Reviewers Colleagues Students and post-docs University administrators People like you Anyone with an interest in what NSF funds We take a look at things Proactive reviews Research Misconduct (RM) Federal-wide definition and procedural framework. RM means fabrication, falsification, or plagiarism in proposing or performing research [], reviewing research proposals [] or in reporting research funded by [the agency]. 45 C.F.R. 689.1.a Fabrication: making up data or results and recording or reporting them Falsification: manipulating materials, equipment, or processes, or changing or omitting data or results Plagiarism: appropriation of another person’s ideas, processes, results or words without giving appropriate credit. The misconduct must have been done with at least reckless intent. The act must be a “significant departure from accepted practices.” Honest error and mistake are not research misconduct. Preponderance of the evidence The RM Procedure Allegation Inquiry Investigation Decision The Referral Process Inquiries and Investigations may be referred to the institution NSF Grant Conditions If institution determines that an investigation is needed it MUST notify NSF immediately RM Case Examples from the NSF OIG Semi-Annual Report to the Congress Falsification, Fabrication, and Plagiarism Found in a Single Proposal (March 2005) PI Ignores Warning to Remove Plagiarized Text From His Proposal (March 2006) PI’s Pattern of Plagiarism Continues During OIG Investigation of His Proposals (March 2006) PI Provides False Evidence to Refute Allegation of Plagiarism (March 2006) Plagiarism Found in University Professor’s Dissertation (March 2006) More RM Case Examples . . . NSF Funded Postdoctoral Fellow Falsifies Research Data (September 2006) Professor Reviews Proposal for NSF, Then Plagiarizes It Into His Own Proposal (Sept 2007) Professor Plagiarizes in Four NSF Proposals (Sept 2007 x 2) Student Claims “Laziness” Caused Him to Fabricate/Falsify Data in Four Manuscripts (Sept 2007) Research Misconduct allegations, investigations, and findings are on the rise. WHY? www.lostinspacetv.com DANGER, Will Robinson, DANGER Useful Tidbits about RM Copyright permission/public domain has nothing to do with plagiarism Text or ideas may be copied Even paraphrasing requires citation “NSF expects strict adherence to the rules of proper scholarship and attribution” for the whole proposal The author should be named in the proposal somewhere All authors share the credit or allegation equally unless evidence shows otherwise More Useful Tidbits about RM Avoid “cleaning up” the figures If the editor requires it, get it in writing Report the “enhancements” in the paper/proposal Review your students’/postdocs’ data Keep good records / notebooks Keep raw data Other Regulatory and Rule Violations Human Subjects / Animal Welfare Violation of Reviewer Confidentiality Annual Financial Conflicts Disclosures Mismanagement of Funds Program Income Participant Support Travel-related issues Time and effort reporting Examples of Other Regulatory Violations Human Subjects / Animal Welfare Cross-discipline research with humans Example: A physical sciences award with an education component for undergraduates that tracks student career paths post-graduation IACUC and IRB Committees w/o assurances Example: Institution with both an IRB and IACUC without approved assurances loses award. When Administrative cases turn Civil/Criminal . . . Case 1: Plagiarizing the Thesis PI submitted his student’s thesis chapter as an SBIR-1 proposal ($100K, 6 months) from a non-existent company. When awarded, PI used the money to pay his child’s tuition at an ivy league institution and other personal expenses. PI copied the thesis into his final report and proposal for the SBIR-2 award ($500K). University notifies OIG of plagiarism allegation PI denied everything. BUT His wife admitted everything When Administrative cases turn Civil/Criminal . . . Case 1: Plagiarizing the Thesis (cont.) NSF suspended the award OIG issued subpoenas. OIG referred the case to DOJ, who accepted it for prosecution. When Administrative cases turn Civil/Criminal . . . Case 1: Plagiarizing the Thesis (cont.) At a meeting with DOJ, the professor through his attorneys agreed 1) Plead guilty to a criminal count (1001) but wanted to avoid jail 2) Would pay $240,000 3) No action against wife NSF OIG recommended RM finding and debarment. Professor and NSF settled for 3 years voluntary exclusion from Federal funding. When Administrative cases turn Civil/Criminal . . . Case 2: “No” Means “No” PI asked NSF program officer for a second No Cost Extension on the Award. NSF program officer declined the request in FastLane, in writing, and by telephone. PI directed the finance officer to draw-down the award balance of $32K although the costs had not been incurred. FINAL OUTCOME: PI was debarred for five years. Institution also paid NSF $52,000+ in restitution and investigative costs. “NSF Proposes to Debar PI for Five Years” (NSF OIG SA Sept 2007) Civil and Criminal Misconduct from the NSF OIG Semi-Annual Report: Former Professor Indicted for Mail, Wire Fraud (March 2006) Embezzlement Investigation Uncovers Additional Issues with the University’s Cost-Sharing and Award Accountability (March 2006) Improperly Used Participant Support Funds Refunded to NSF (September 2006) Former Research Center Employee Sentenced to Prison for Mail Fraud (September 2007) Civil and Criminal Misconduct: Common Issues False Statements / False Claims Certifications are especially important Criminal sanctions – fines and jail Civil sanctions – up to treble damages possible Mail / Wire Fraud NSF FastLane system What we don’t do General scientific ethics Academic Divorces Institutional personnel issues that do not violate statutes, regulations, or grant conditions connected with NSF programs Authorship disputes i.e., Whose name goes on the paper? In what order? However omission of a name could be intellectual property theft, which we do investigate. Where do you fit in? Investigation Committee Member? Witness? Subject? Investigation Committee (IC) Member Institution usually appoints a committee Committee member obligations Follow the institutional policy Explain the decision as supported by the evidence Confidentiality Avoid the Faculty / Student double standard IC Issues #1 Institution conducted investigation finding that the PI had knowingly and recklessly plagiarized over 150 lines of text from over 20 sources = “violation of institutional standard of scholarly integrity” BUT said it was not a “significant departure from accepted practices in the research community” as it was “low level copying” IC Issues #2 Institution conducted inquiry into data fabrication and notified NSF OIG that it was proceeding with an investigation. We concurred, referred, and deferred. Subject got an attorney; Institution changed mind citing many plausible explanations for data RESULT: OIG in-house investigation. Subject debarred for 2 years, plus other requirements before he can receive NSF funds again. IC Lessons Learned The report and recommendations should be supported by logic and evidence Don’t decide first and then fit the evidence to the decision Don’t let an attorney intimidate or confuse you You have a University GC – make the GC earn his/her keep It’s not prime time TV – You don’t have to solve it in an hour Inquiries are to determine whether an investigation is needed. Investigations are when you collect all the information. Generally, its preponderance of the evidence that’s needed. Witness? Complainants are witnesses, not plaintiffs Witness obligations CONFIDENTIALITY TRUTHFULNESS Participation is voluntary and confidential to the extent possible Subject? Allegations are unsubstantiated rumors Firewall between OIG and program office to prevent taint When possible in RM cases, subject is contacted first Protect reputations until there is a determination If you go to your GC, understand his/her obligations Think before you speak If the excuse doesn’t fly when your student uses it ... Your parents were right: Tell the truth and the process goes much easier What happens at the end of the investigation? Matter could close for lack of evidence Majority of allegations If sufficient evidence: OIG reports to the decision maker: DOJ for Civil/Criminal NSF Office of the Director for RM and other regulatory issues OIG makes recommendations Possible Outcomes Whatever sanctions the institution makes Letter of Reprimand Ban from serving as a reviewer Ethics Training Certifications Assurances Federal-wide Debarment Fines / Restitution Prison Contact Information www.oig.nsf.gov Hotline:1-800-428-2189 E-mail:oig@nsf.gov Fax:(703) 292-9158 Mail: 4201 Wilson Boulevard Suite II-705 Arlington, VA 22230 ATTN: OIG HOTLINE Scott J. Moore, Ph.D., J.D. Investigative Scientist smoore@nsf.gov 703-292-4991 QUESTIONS?