Doonesbury, 5 December 2005
advertisement

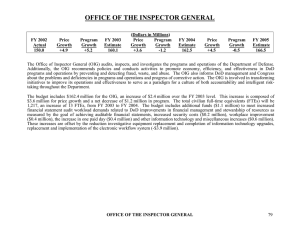
Doonesbury, 5 December 2005 Institutional Compliance Plans Why before is better than after Scott J. Moore, Ph.D., J.D. Investigative Scientist smoore@nsf.gov 703-292-4991 Offices of Inspectors General • Provide leadership and coordination to implement policies to: • Prevent and detect fraud, waste, abuse • Promote economy, efficiency, effectiveness • Conduct investigations, audits, inspections, reviews of agency programs (funded activities), operations • Features: • Independent of agency management • Jurisdiction (NSF activities, programs, operations) • Staff of experts: administrators, attorneys, auditors, criminal investigators, and scientists The Basics A system of responsible administrative, financial, and research management and oversight, creating an environment in which employees can operate with integrity. May be implemented voluntarily or mandated as part of negotiated resolution Does your organization have what it takes? Leadership - commitment to do the right thing Management - ethical environment Focus on high risk areas Provide systematic monitoring, auditing, oversight Training - Communicate facts and expectations Action - Early detection and correction of problems Reporting – Relay information regarding wrongdoing Effective Compliance Program Elements* a/k/a 7 Habits of Highly Effective Organizations Reasonable Compliance Standards and Procedures Specific High-level Personnel Responsible Due Care in Assignments with Discretionary Authority Effective Communication of Standards and Procedures Establish Monitoring and Auditing Systems and Reporting System (whistleblowing without fear of retaliation) Consistent Enforcement of Standards through Appropriate Mechanisms (including failure to detect) Respond Appropriately to the Offense (reporting to law enforcement, modify program, prevention) *Federal Sentencing Guidelines U.S.S.G. §§ 8B2.1, 8C2.5(f), & 8D1.4(c)(1) (11/1/04) Risk (and how to find it) Auditors Squeaky Wheels Whistleblowers Investigators ARE your friends One University’s Risk Assessment Heat Map High PROBABILITY Privacy of Information Deferred Maintenance Environmental, Health & Safety Human Auxiliary Services Subjects Off-Campus Facilities Moderate Executive Benefits Contracts & Grants Management Immigration & Visa Processing Student Affairs Property Management Human Resource Management Endowment Animal Subjects Gifts & Restricted Funds Acquisition Security Construction Management Financial Reporting Technology Licensing Low Low Moderate IMPACT High Finding Risk What needs more review? Where’s your itch? What’s autonomous? Where’s the black hole? Are your warning bells ringing when you hear someone else’s problems? What gives you that “Gosh, gotta check that right away” feeling? Risk Areas Allowable activities supported Allowable costs and cost principles Cash management Eligibility for awards Equipment and real property management Period of availability of funds Procurement suspension and debarment Program income Participant support Timely required reporting Special tests and provisions Holding accounts Summer salaries Other Risk Areas Lack of adequate documentation travel documentation cost-sharing records retention credit card receipts do not constitute adequate documentation Time and effort reporting and procedures Separate financial administration for each award, no pooling Abuse or violations of institutional conflict of interest disclosure policies and procedures. Updated/adequate RM policies and procedures Subawardee monitoring (and A-133s) Residual funds Oversight activities (Conflict of Interest, Humans, Animals) What do you look like? Funding Characteristics Grants, contracts and with what entity Organizational Profile Decentralized Control Profile Rainmakers, President, staff Audit Profile How easily can you get down into the weeds? Activities Location On-site, off-site, another country, LTER, SBIR, SGER Activity Description Animals, humans, collection, collaboration, toxins, radiation; staffing characteristics “Junior, independent” or “senior and wise” Training Profile Comprehensive administrative, financial, oversight, fit the issues Common Types of Civil/Criminal Allegations 3% 9% 31% Theft/Embezzlement (31%) False or Fraudulent Statements (24%) 13% Miscellaneous* (20%) False or Fraudulent Claims (13%) Conflicts of Interest (9%) 20% Computer Fraud (3%) 24% *Includes mail fraud, false identification insurance fraud, impersonating a government officer, and copyright infringement. Data gathered from NSF OIG closed Investigative files (1990 – Present). Investigation Outcomes 11% 8% Unsubstantiated Allegations* (32%) 8% Restitution (21%) 21% 6% Referral (11%) Termination of Grant (8%) Miscellaneous** (8%) 6% Incarceration/Probation (6%) Termination of Employment (6%) 4% 4% Civil Action (4%) Debarment (4%) 32% * Allegation was preliminarily investigated but found to be insufficiently material to warrant further action. ** Includes monitoring, enacting new guidelines, letters of reprimand, etc. Data gathered from NSF OIG closed Investigative files (1990 – Present). Common Issues Cost Share Program Income Subrecipient Monitoring Effort Reporting Participant Support Travel Signature Responsibilities “GRANTS GONE BAD” Proposal Signatures Compliance with award terms and conditions Accuracy and completeness of statements COI Policy Drug-Free Workplace Debarment and Suspension Lobbying (proposal >$100,000) Certification for Authorized Organizational Representative or Individual Applicant: By signing and submitting this proposal, the individual applicant or the authorized official of the applicant institution is: (1) certifying that statements made herein are true and complete to the best of his/her knowledge; and (2) agreeing to accept the obligation to comply with NSF award terms and conditions if an award is made as a result of this application. Willful provision of false information in this application and its supporting documents or in reports required under an ensuring award is a criminal offense (U. S. Code, Title 18, Section 1001). In addition, if the applicant institution employs more than fifty persons, the authorized official of the applicant institution is certifying that the institution has implemented a written and enforced conflict of interest policy that is consistent with the provisions of Grant Policy Manual Section 510; that to the best of his/her knowledge… . . . Debarment Certification Certification Regarding Lobbying Certification for Contracts, Grants, Loans and Cooperative Agreements AUTHORIZED ORGANIZATIONAL REPRESENTATIVE SIGNATURE DATE Page 2 of 2 “Well, I’m in the clear. I certainly didn’t KNOW that certification was false when I signed it.” No Problems, Right? Knowing and Knowingly Defined 31 U.S.C. 3729(b) 1) acts with actual knowledge of the information; 2) acts in deliberated ignorance of the truth or falsity of the information; or 3) acts in reckless disregard for the truth or falsity of the information. 4) And, no proof of specific intent to defraud is required. Examples of Misused Funds 1. 2. 3. 4. 5. Grant Fraud Embezzlement Multi-Agency Fraud False Certifications Overcharging Grants Case Study 1: “No” Means “No” NSF OIG conduct a Proactive Review Proactive Review identified questionable entries in General Ledger of a small institutional awardee. Investigation initiated Obtained and reviewed all financial records Identified improper drawdown Revealed $27K expenditures incurred after expiration of award Case Study 1: “No” Means “No” (cont) OIG investigation of those expenditures revealed that PI requested award extension to spend remaining funds Request was denied 3X by Program Officer Nevertheless, after award expiration, PI drew down and spent the $27K on post-expiration expenses PI falsely characterized drawdown as reimbursements PI filed Final Project Report falsely reflecting program completion PI and awardee made false statements to OIG – claimed lack of knowledge regarding Program Officer’s 3 denials of request Case Study 1: “No” Means “No” (cont) Presented case to AUSA (civil and criminal) Proceeded as civil false claims case DOJ settled case Imposed a 3-year Compliance Plan NSF OIG recovered $52K from institution NSF debarred PI for 5-years Case 2: “Just Charge It To The Grant With Money In It” A company received a grant from NSF A source provided information to NSF OIG, alleging that the company charged time to the NSF grant for work she did not perform NSF OIG opened investigation Interviewed company officials Interviewed employees Obtained and reviewed records Case 2: “Just Charge It . . .” (cont) OIG investigation revealed some of the time charged to the grant should have been charged to overhead the company shifted charges between various federal grants as money was available the company charged grants for proposal preparation the company charged grants for costs unrelated to the grant NSF was overcharged $370,000 other federal agencies were overcharged $970,000 Case 2: “Just Charge It . . .” (cont) Presented case to AUSA Proceeded as civil false claims case DOJ settled case $1.55M 4-year Compliance Plan imposed Case 3: Know what you are signing Allegation received: A Principal Investigator complained that he was not receiving the cost-share monies he was promised by the university Investigation opened: Request and review financial records for grant Travel to university Interview relevant individuals Coordinate with Department of Justice Case 3: Know what you are signing (cont) Investigation revealed: the university did not have a system for tracking cost-share could not document costs submitted to NSF as cost-share could not document costs claimed as direct expenses the university had submitted numerous false cost-share certifications Case 3: Know what you are signing (cont) Presented case to AUSA Proceeded as civil false claims case DOJ settled case Imposed a 5-year Compliance Plan NSF OIG recovered $1.4M Case 4: Plagiarizing the Thesis a/k/a Watch out for the CPS and COI PI submitted his student’s thesis chapter as an SBIR-1 proposal ($100K, 6 months) from a non-existent company. When awarded, PI used the money to pay his child’s tuition at an ivy league institution and other personal expenses. PI copied the thesis into his final report and proposal for the SBIR-2 award ($500K). University notifies OIG of plagiarism allegation PI denied everything. BUT His wife admitted everything Case 4: Plagiarizing the Thesis a/k/a Watch out for the CPS and COI NSF suspended the award OIG issued subpoenas. OIG referred the case to DOJ, who accepted it for prosecution. Case 4: Plagiarizing the Thesis a/k/a Watch out for the CPS and COI At a meeting with DOJ, the professor through his attorneys agreed 1) 2) 3) Plead guilty to a criminal count (1001) but wanted to avoid jail Would pay $240,000 No action against wife NSF OIG recommended RM finding and debarment. Professor and NSF settled for 3 years voluntary exclusion from Federal funding. Could a proactive compliance plan have prevented the loss? References http://oig.hhs.gov/fraud/complianceguidance.html http://www.nacua.org/documents/FedSentencingGuidelines.pdf http://www.ussc.gov/corp/Murphy1.pdf http://www.usdoj.gov/dag/cftf/corporate_guidelines.html Grant, G. Odell, G., and Forrester, R; Creating Effective Research Compliance Programs in Academic Institutions; Academic Medicine, Vol 74, No. 9, September 1999, p. 951. Jordan, K. and Murphy, J.; Compliance Programs: What the Government Really Wants. p. 121. Managing Externally funded Research Programs; A Guide to Effective Management Practices; Council on Government Relations, June 2005 DHHS Draft OIG Compliance Program Guidance for Recipients of PHS Research Awards; Fed. Reg. Monday Nov 28, 2005, vol. 70#227, p:71312 Ingram, Ginna; Corporate Compliance Programs: More Than Window Dressing. Journal of Public Inquiry, Spring/Summer 2007, p. 5. http://www.ignet.gov/randp/sp07jpi.pdf Contact Information www.oig.nsf.gov Hotline:1-800-428-2189 E-mail:oig@nsf.gov Fax:(703) 292-9158 Mail: 4201 Wilson Boulevard Suite II-705 Arlington, VA 22230 ATTN: OIG HOTLINE Scott J. Moore, Ph.D., J.D. Investigative Scientist smoore@nsf.gov 703-292-4991 ?