CHEMISTRY 1 Term 1, 2012 – 12:00pm Tuesdays 9:00am
advertisement

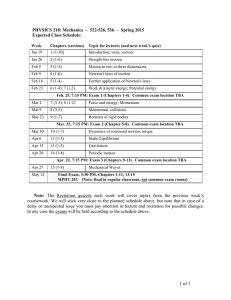
Dan Solomon | Phone 4348 4326 | email: daniel.solomon@tafe.nsw.edu.au CHEMISTRY 1 Term 1, 2012 Tuesdays 9:00am – 12:00pm The formal assessment events for this subject consist of two (2) written exams. A pass mark of 50% or greater must be achieved to pass this subject. Class worksheets are considered an informal component of your assessment. It is highly recommended these tasks are attempted and submitted where applicable, as a means of gaining feedback of your progress in this subject. Compulsory prescribed text: Chemistry for Technicians - Barker, Fullick and Krajniak. The timetable of concepts covered, as appears below, serves to act as a guide of subject progress. Date Theory 7 Feb Introduction, Chapter 1: Classification of Matter 14 Feb Chapters 2, 3, 7: Atomic Structure, Periodic Table 21 Feb Chapters 3, 7, 9: Periodic Table, Atomic Bonding 28 Feb Chapters 9, 4: Atomic Bonding, Names and Chemical Formula 6 Mar Chapters 4, 8: Names and Chemical Formula 13 Mar EXAM 1 (closed book, A4 cheat sheet permitted ) 20 Mar Chapter 5: Properties and Structure 27 Mar Chapters 6, 11: Chemical Reactions 3 Apr Chapters 6, (4): Moles and Yields 10 Apr 24 April Practical (TPC Chem A, 2012) States of matter (pp 8-11) Techniques for separating mixtures (pp 31-32). Making observations Properties and structure: Investigating elements (pp 44-49) Physical versus chemical change (p29) Modelling molecules and compounds (pp 64-65) Properties and structure: Physical properties of compounds (pp71) Solubility, precipitation reactions and ionic equations (pp90-91) Demo – mole quantities Yield Expt: Magnesium Oxide (p102) HOLIDAY Chapter 10: Concentrations, Molarity and Dilutions 1 May Catchup and REVISION 8 May EXAM 2 (closed book, A4 cheat sheet permitted) This subject contributes to meeting course requirements according to units of competence comprised within the course you have undertaken. Cert 3 results for this subject will correspond to the unit identified on your transcript: “Prepare working solutions”. Cert 4 results for this subject will correspond to the unit identified on your transcript: “Prepare, use and standardise solutions”. Chemistry of Natural Products 13/02/2012