fide Reactivity in the Detection of Protein S‑Sulfhydration Persul *
advertisement

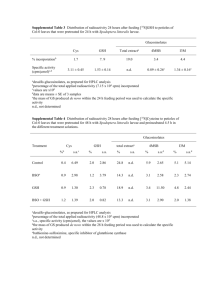
Letters pubs.acs.org/acschemicalbiology Persulfide Reactivity in the Detection of Protein S‑Sulfhydration Jia Pan and Kate S. Carroll* Department of Chemistry, The Scripps Research Institute, Jupiter, Florida 33456, United States S Supporting Information * ABSTRACT: Hydrogen sulfide (H2S) has emerged as a new member of the gaseous transmitter family of signaling molecules and appears to play a regulatory role in the cardiovascular and nervous systems. Recent studies suggest that protein cysteine Ssulfhydration may function as a mechanism for transforming the H2S signal into a biological response. However, selective detection of S-sulfhydryl modifications is challenging since the persulfide group (RSSH) exhibits reactivity akin to other sulfur species, especially thiols. A modification of the biotin switch technique, using S-methyl methanethiosulfonate (MMTS) as an alkylating reagent, was recently used to identify a large number of proteins that may undergo S-sulfhydration, but the underlying mechanism of chemical detection was not fully explored. To address this key issue, we have developed a protein persulfide model and analogue of MMTS, S-4-bromobenzyl methanethiosulfonate (BBMTS). Using these new reagents, we investigated the chemistry in the modified biotin switch method and examined the reactivity of protein persulfides toward different electrophile/ nucleophile species. Together, our data affirm the nucleophilic properties of the persulfide sulfane sulfur and afford new insights into protein S-sulfhydryl chemistry, which may be exploited in future detection strategies. O results, the structure of reaction products in each step of the modified BST and the underlying mechanism of thiol vs persulfide MMTS selectivity was not investigated. Herein, we report several persulfide models and new MMTS analogue, S-4-bromobenzyl methanethiosulfonate (BBMTS). Using these reagents, we investigated the chemistry in the modified BST and examined the reactivity of protein persulfides toward different electrophile/nucleophile species. These data reaffirm the nucleophilic properties of the persulfide sulfane sulfur and afford new insights into protein S-sulfhydryl chemistry, which may be exploited in future detection strategies. Although selective (albeit slow) methods for H2S detection have rapidly accumulated,15−18 methods for detecting protein persulfide modifications have been slower to advance. In this case, the challenges are 2-fold: (i) the chemical reactivity of the terminal sulfane sulfur in the protein persulfide is similar to the thiol and (ii) few examples of robust S-sulfhydryl protein models have been reported. To this end, we initiated studies to explore the generation and reactivity of protein persulfides. Our second objective was to understand the chemical mechanisms xidative post-translational modification (oxPTM) of protein cysteines plays an important regulatory role in many physiological systems.1−3 Of these, protein S-sulfhydration has recently received attention in the context of hydrogen sulfide (H2S) mediated signaling pathways4−7 involved in cardiovascular and cerebral vasodilatory reponses.8,9 To date, the precise mechanisms of H2S action are still the subject of active investigation. A significant challenge in protein Ssulfhydration research is that the persulfide group (RSSH) exhibits reactivity akin to other sulfur species, in particular thiols,10−12 which compounds difficulties in developing selective detection methods. In regards to detection, our interest was piqued when a commonly used alkylating agent, known as S-methyl methanethiosulfonate (MMTS),13 was reported to differentiate thiols and persulfides using a modified biotin switch technique (BST),4 originally designed to study protein S-nitrosylation.14 As shown in Figure 1a, in the modified BST thiols are first alkylated with MMTS, and persulfides were postulated to remain unreacted and be available for subsequent conjugation to N-[6-(biotinamido) hexyl]-3′-(2′-pyridyldithio) propionamide (biotin-HPDP). Using this modified version of the BST, a large number of proteins were reported as targets of H2S in the mouse liver, and the basal sulfhydration level of some proteins was estimated to be as high as 25%.4 Despite these intriguing © XXXX American Chemical Society Received: February 13, 2013 Accepted: April 4, 2013 A dx.doi.org/10.1021/cb4001052 | ACS Chem. Biol. XXXX, XXX, XXX−XXX ACS Chemical Biology Letters Figure 1. Reported methods for detecting protein persulfide modfiications. (a) Modified biotin switch technique (BST): protein samples are alkylated by MMTS to block free thiols, while sparing persulfides. In the next step, biotin-HPDP is applied to alkylate persulfides and form protein− biotin conjugates. (b) Thiols and persulfides are reacted with iodoacetic acid (IAA). The persulfide is then reduced with dithiothreitol (DTT) and conjugated to biotinylated IAP. (c) Other cysteine oxPTMs that may be identified using the method in b. Figure 2. Preparation and verification of persulfide formation in GSH and protein models. (a) Formation of glutathione (GSH) persulfide. (b) HPLC UV trace at 254 nm and corresponding masses from the reaction in a. (c) Formation of protein persulfides. Mass analysis of the reaction between iodoacetamide (IAM), (d) papain persulfide, and (e) Gpx3 persulfide [*indicates the product derived from alkylation of Gpx3−S−S−SH (i.e., trisulfide)]. B dx.doi.org/10.1021/cb4001052 | ACS Chem. Biol. XXXX, XXX, XXX−XXX ACS Chemical Biology Letters underlying current chemoproteomic methods of persulfide detection. In an effort to identify suitable models, we first investigated the reported method19 for preparing persulfide derviatives of the tripeptide glutathione. As illustrated in Figure 2a, the GSH persulfide was obtained as an equilibrium mixture of glutathione thiol (GSH, 2) and persulfide (GSSH, 3) after the reaction of glutathione disulfide (GSSG, 1) with Na2S. Though GSH persulfide 3 could not be directly observed by liquid chromatography−mass spectrometry (LC−MS), its formation was confirmed through conjugation with alkylating agents such as iodoacetamide (IAM) and N-ethylmaleimide (NEM), which yielded detectable products in LC−MS analyses (Figure 2b and Supplementary Figure S1). The inability to directly observe the persulfide species likely stems from the instability of the underivatized modification in acidic conditions and/or poor ionization properties. In parallel investigations, single protein persulfide modifications were generated via a stepwise procedure in the cysteine protease, papain, and the C64,82S thiol peroxidase, Gpx3. In this method, reduced thiol 6 was first reacted with 5,5′dithiobis-2-nitrobenzoic acid (DTNB or Ellman’s reagent) to form activated disulfide 7, which was then cleaved by Na2S to generate persulfide 8 (Figure 2c). After conditions such as the equivalents of DTNB and Na2S were optimized, papain and Gpx3 persulfides were successfully prepared and confirmed by intact MS analysis post-trapping with IAM or NEM (Figure 2d). In addition to the desired papain persulfide derivative 9, we observed signals consistent with papain sulfinic acid 10 (asisolated from the original commercial sample) as well as persulfide oxidation. Of note, these species were also observed in the reaction with NEM (Supplementary Figure S2), indicating these mass signals were not unique to a particular alkylating reagent. Interestingly, in the case of Gpx3, the alkylation reaction revealed the presence of both the persulfide (major derivative) and the trisulfide (minor derviative; Figure 2e and Supplementary Figure S3). Next, we tested the reaction of the persulfide models with MMTS. As shown in Figure 3a, the reaction of glutathione persulfide with MMTS gave a small amount of GSSH derivative 14, with major product 15 derived from GSH, and other byproducts GSSG (1) and GSSSG (13). The desired papain persulfide 16 and Gpx3 persulfide 18 products were clearly observed as the major species, albeit with some overlap between protein sulfonates and thiosulfates (i.e., papain 17 in Figure 3b; Gpx3 20 and 21 in Figure 3c). These analyses also highlight the small mass difference (∼2 Da; Figure 3d) between byproducts from the oxidation of thiols and persulfides, relative to the MMTS-modified sulfur, which makes it difficult to distinguish between these chemically distinct species by intact protein MS analysis. To address the above issue and clarify whether the ∼80 Da mass increase corresponds to −SSMe or −SSO3− forms of GSH, papain, and Gpx3, we designed and synthesized an analogue of MMTS, S-4-bromobenzyl methanethiosulfonate (BBMTS; Figure 4a). Compared to MMTS, the advantage of this reagent is 2-fold: (i) a larger mass change results from the thio-BBMTS adduct (+200 Da), and (ii) BBMTS exhibits the telltale bromine mass pattern (i.e., strong peaks at M − 1 and M + 1), which can be discerned in low-molecular-weight peptides, including GSH. Initially, we verified the reactivity of BBMTS, which proved comparable to MMTS (Supplementary Tables S1 and S2). We then tested the BBMTS reagent in Figure 3. Reactivity of GSH and protein persulfide models with MMTS. (a) HPLC UV trace at 254 nm and corresponding masses from the reaction of GSH persulfide and MMTS (*the mass in this peak could not be assigned to a chemical structure). Mass analysis of the reaction between (b) papain persulfide and MMTS and (c) Gpx3 persulfide and MMTS. (d) Increase in molecular mass that results from MMTS modification (−SSMe) or oxidation to the thiosulfate (−SO3−). reactions with the GSH and protein persulfide models. As shown in Figure 4b, treatment of glutathione persulfide with BBMTS, gave a small amount of the desired persulfide derviative 25 and a large peak of the thiol derviative 26 (both confirmed using the bromine signature afforded by the new reagent), as well as the thiosulfate byproduct 27 (also detailed in Supplementary Figures S4−7 and Table S3). However, the reaction between protein persulfides and BBMTS clearly showed the formation of the desired persulfide derivatives, as well as alkyl polysulfides (Figures 4c,d). Taken together, these data unequivocally establish that the nucleoC dx.doi.org/10.1021/cb4001052 | ACS Chem. Biol. XXXX, XXX, XXX−XXX ACS Chemical Biology Letters Figure 4. Reactivity of the GSH and protein persulfide models with BBMTS. (a) Synthesis of S-4-bromobenzyl methanethiosulfonate (BBMTS). Mass analyses of the reaction between (b) GSH persulfide and BBMTS, (c) papain persulfide and BBMTS [+papain−SO3− (sulfonic acid); *papain−S−SO3− (thiosulfate)], and (d) Gpx3 persulfide and BBMTS. Figure 5. Sequential labeling reactions of protein persulfides with MMTS or BBMTS and biotin-HPDP analogue, N-acetylcysteine pyridyldisulfide (NACP). Mass analyses of the reaction between (a) papain persulfide, MMTS and then NACP, b() papain persulfide, BBMTS, and then NACP [+papain−SO3− (sulfonic acid); *papain−S−SO3− (thiosulfate)], (c) Gpx3 persulfide, MMTS, and then NACP [*Gpx3−S−S-S-SMe (tetrasulfide)], and (d) Gpx3 persulfide, BBMTS, and then NACP as described in the Methods section [*Gpx3−S−S-S−SCH2C6H4Br (tetrasulfide)]. philic sulfane sulfur of protein persulfides reacts with electrophilic MMTS and BBMTS reagents. Since the MMTS and BBMTS electrophiles react with protein persulfides, an important question arises: what does the biotin signal in the modified BST stem from? To address this issue, we treated papain and Gpx3 products of the MMTS/ BBMTS alkyation step with the biotin-HPDP analogue, Nacetylcysteine pyridyldisulfide (NACP). Ensuing intact MS analysis of these reactions indicated the following: (i) no product corresponding to a NACP-labeled persulfide was detected in papain or Gpx3, (ii) a small amount of NACPlabeled thiol was observed in papain (32; Figures 5a,b), and (iii) no NACP-labeled thiol was observed in Gpx3 (Figures 5c,d). These studies suggest that the protein-NACP labeling product is derived from thiol alkylation. Finally, since little has been reported about the chemical reactivity of protein persulfides, we investigated the behavior of the Gpx3-SSH sulfane sulfur with various nucleophilic and D dx.doi.org/10.1021/cb4001052 | ACS Chem. Biol. XXXX, XXX, XXX−XXX ACS Chemical Biology Letters Table 1. Reactivity of Gpx3 Persulfide Towards Electrophilic and Nucleophilic Reagents carbon nucleophiles (Supplementary Figures S9 and S13F−K and Table S4). We have investigated GSH and protein persulfide models for chemical reactivity studies. Among these prototypes, GSH and papain persulfides have been previously described; 19 however, the protein persulfides were not evaluated or confirmed by mass analysis. Using this early study as inspiration, in this work, we have examined the utility of a new persulfide protein model, based on a single-cysteine mutant of thiol peroxidase, Gpx3. This model proved quite facile for the generation of protein persulfides, and intact MS analysis was not complicated by the presence of a large fraction sulfinic acid-modified protein, in contrast to that observed for commercial sources of papain. With respect to the GSH persulfide model, we observed only a small amount of persulfide IAM and NEM alkylation products. The most likely explanation for these data is that the reaction of sterically unhindered persulfide derviatives 14 and 25 with low molecular weight thiols (i.e., GSH and GSSH) is rapid, relative to chemical alkylation. Thus, compared to protein persulfide electrophilic reagents (Table 1 and Supplementary Figure S8). The nucleophilicity of the sulfane sulfur was reaffirmed in reactions with electrophilic alkylating reagents such as IAM, NEM, and MMTS (Entry 1−3), as well as disulfide-containing compounds, like DTNB and NACP (Entry 4−5). As an electrophile, the sulfane sulfur could be reduced to the free thiol form by DTT (Entry 6), GSH (note free thiol and mixed disulfide forms; Entry 7), and NAC (Entry 8). By contrast, weaker sulfur-based nucleophiles like sulfite or methyl sulfinate did not reduce the Gpx3 persulfide (Entry 9−10). We also tested the reaction of Gpx3 persulfide with carbon nucleophiles known to react with sulfenic acid (RSOH), but not disulfides or nitrosothiols (RSNO). These studies show that dimedone, malononitrile, methyl acetoacetate, and barbituric acid carbon nucleophiles exhibited no reactivity toward the Gpx3 persulfide (Entries 11−14). As before, thiosulfate formation was also noted in the persulfide samples, most likely resulting from oxidation by trace metal ions in the presence of oxygen.20 The thiosulfate species was stable and also did not react with the E dx.doi.org/10.1021/cb4001052 | ACS Chem. Biol. XXXX, XXX, XXX−XXX ACS Chemical Biology Letters Figure 6. Proposed labeling mechanism of protein persulfide, methanthiosulfonate (MMTS or BBMTS), and pyridydisulfide NACP. Two possible models are possible. (a) Free thiols may be incompletely blocked in the first MMTS alkylation step and subsequently react with the pyridyldisulfide biotin reagent. (b) Alternatively, or in addition, labeling may be achieved via stepwise thiol-disulfide exchange in a reaction catalyzed by trace free thiols (RSH). thiols. Likewise, the γ-sulfur of persulfide is a weak electrophile that shares some reactivity with disulfides (also alluded to by sulfur transfer mechanisms in Fe−S biosynthetic pathways12) but is chemically distinct from the sulfur atom in sulfenic acid, which is significantly more electrophilic in nature. Collectively, these data reaffirm the nucleophilic properties of the persulfide sulfane sulfur and afford new insights into protein S-sulfhydryl chemistry, which can serve as a resource in developing future detection strategies. models, the GSH persulfide may have less utility in reactivity studies. In addition, we have examined the chemistry underlying the derivitization steps in the modified BST using the GSH and protein persulfide model systems. In contrast to earlier supposition,4 we demonstrate that the terminal persulfide sulfur undergoes facile reaction with both MMTS and BBMTS and that these alkylated forms do not react with the NACP pyridyldisulfide. These findings thus reaffirm the nucleophilic reactivity of the sulfane sulfur atom in protein persulfides and are consistent with physical organic studies10,11 and the IAM/ NEM labeling observed in this work. In fact, the only NACPlabeled species that we observed in this study corresponded to the reaction product derived from thiol, as opposed to the persulfide. As shown in Figure 6, two possible models could account for this finding. In one scenario, free thiols may be incompletely blocked in the first MMTS alkylation step and subsequently react with the pyridyldisulfide biotin reagent (Figure 6a). Alternatively, or in addition, biotin labeling may be achieved via stepwise thiol-disulfide exchange in a reaction catalyzed by trace free thiols (Figure 6b). A common element in both models is that not all free thiols are blocked during the MMTS labeling step. Recently, it was also reported that the persulfide-modified active site cysteine of protein tyrosine phosphatase 1B (PTP1B) can be alkylated by iodoacetic acid (IAA). Subsequent reduction of the persulfide adducts and labeling with iodoacetamide-linked biotin (IAP) has also been proposed as an indirect approach for detecting protein S-sulfhydration (Figure 1b).6 However, it is not apparent how this method distinguishes persulfide modifications from other DTTreducible modifications, such as disulfide bonds, sulfenic acids, and nitrosothiols (Figure 1c). Neverthless, IAA derivitization of the PTP1B persulfide provides further evidence that the persulfide is nucleophilic with similar reactivity to ■ METHODS Sodium sulfide nonahydrate (Na2S·9H2O), extra pure, was obtained from ACROS. DTNB was obtained from Sigma, and the purity was ≥98%. Papain 2× crystallized was purchased from Sigma. All other chemicals were purchased from Sigma-Aldrich or Fisher at the highest available purity. Preparation of Persulfides. (1). Glutathione Persulfide.19 To a solution of freshly prepared glutathione (oxidized form, 1 mM final concentration) in 1 mL of Tris-HCl buffer (100 mM, pH 7.4) was added freshly prepared sodium sulfide (1 mM final concentration), and the solution was stirred at 37 °C for 15 min. The resulting glutathione persulfide (1 mM final concentration) was directly used without further purification. (2). Papain Persulfide.19 A solution of papain (10 mg) in 1 mL of Tris-HCl (pH 7.4, 100 mM, degassed with N2 for 10 min) was incubated with cysteine (1.7 mg) at rt for 10 min, and the protein was purified through a PD10 column (GE Healthcare) containing G-25 Sephadex. Protein-containing fractions were pooled from this column (500 μL, 270 μM) and incubated with DTNB (50 μL, 4 mM) at rt for 20 min, and purified by passing through a PD-10 column. A fraction of the pooled protein (200 μL, 120 μM) was then incubated in a solution of Na2S (4 μL, 30 mM) at rt for 10 min and purified by PD-10 column to give the papain persulfide, which was immediately used in further reactions. (3). Gpx3 Persulfide. C64,82S Gpx3 was prepared as previously described.21,22 A solution of C64,82S Gpx3 (100 μM, 100 μL) was buffer exchanged through a P-30 spin filtration column (BioRad) that F dx.doi.org/10.1021/cb4001052 | ACS Chem. Biol. XXXX, XXX, XXX−XXX ACS Chemical Biology Letters sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 45, 13−24. (8) Zhao, W., Zhang, J., Lu, Y., and Wang, R. (2001) The vasorelaxant effect of H2S as novel endogenous gaseous gaseous KATP channel opener. EMBO J. 20, 6008−6016. (9) Olson, K. R., Dombkowski, R. A., Russell, M. J., Doellman, M. M., Head, S. K., Whitfield, N. L., and Madden, J. A. (2006) Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 209, 4011− 4023. (10) Flavin, M. (1962) Microbial transsulfuration: the mechanism of an enzymatic disulfide elimination reaction. J. Biol. Chem. 237, 768− 777. (11) Heimer, N. E. (1981) Biologically oriented organic sulfur chemistry. 21. Hydrogendisulfide of a penicillamine derivative and related compounds. J. Org. Chem. 46, 1374−1377. (12) Mueller, E. G. (2006) Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2, 185−194. (13) Smith, D. J., Maggio, E. T., and Kenyon, G. L. (1975) Simple alkanethiol groups for temporary blocking of sulfhydryl groups of enzymes. Biochemistry 14, 766−771. (14) Jaffrey, S. R., and Snyder, S. H. (2001) The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 86, l1. (15) Lippert, A. R., New, E. J., and Chang, C. J. (2011) Reactionbased fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 133, 10078−10080. (16) Liu, C., Pan, J., Li, S., Zhao, Y., Wu, L. Y., Berkman, C. E., Whorton, A. R., and Xian, M. (2011) Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew. Chem., Int. Ed. 50, 10327−10329. (17) Qian, Y., Karpus, J., Kabil, O., Zhang, S., Zhu, H., Banerjee, R., Zhao, J., and He, C. (2011) Selective fluorescent probes for live-cell monitoring of sulphide. Nat. Commun. 2, 495. (18) Lin, V. S., and Chang, C. J. (2012) Fluorescent probes for sensing and imaging biological hydrogen sulfide. Curr. Opin. Chem. Biol. 16, 595−601. (19) Francoleon, N. E., Carrington, S. J., and Fukuto, J. M. (2011) The reactivity of H2S with oxidized thiols: Generation of persulfides and implications to H2S biology. Arch. Biochem. Biophys. 516, 146− 153. (20) Everett, S. A., and Wardman, P. (1995) Perthiols as antioxidants: radical-scavenging and prooxidative mechanisms. Methods Enzymol. 251, 55−69. (21) Mason, J. T., Kim, S. K., Knaff, D. B., and Wood, M. J. (2006) Thermodynamic basis for redox regulation of the Yap1 signal transduction pathway. Biochemistry 45, 13409−13417. (22) Paulsen, C. E., and Carroll, K. S. (2009) Chemical dissection of an essential redox switch in yeast. Chem. Biol. 16, 217−225. had been equilibrated in Tris-HCl buffer (pH 7.4, 100 mM, degassed with N2 for 10 min). After determination of protein concentration (80 μM), the solution was incubated with DTNB (4 μL, 4 mM) at rt for 20 min and purified again by spin filtration. The resulting protein (100 μL, 62 μM) was then incubated with a Na2S solution (1 μL, 30 mM) at rt for 10 min and purified by spin column to give the Gpx3 persulfide, which was immediately used in downstream reactions. Verification of Persulfide Formation and Analysis of Chemical Reactivity. Freshly prepared GSH or protein persulfide (final concentration 1 mM for glutathione; 20 μM for protein) was incubated with IAM or other nucleophilic or electrophilic reagents (final concentration 4 mM for glutathione; 2 mM for protein) at rt for 1 h. After purification, aliquots of the samples were analyzed by electrospray LC−MS on a Poroshell 120 (Agilent) or ZIC-pHILIC HPLC column (the Nest group). Reaction of GSSH with MMTS or BBMTS. To a solution of freshly prepared glutathione persulfide (1 mM final concentration) in 1 mL of Tris-HCl buffer (100 mM, pH 7.4) was added MMTS or BBMTS (4 mM final concentration), and the solution was stirred at rt for 30 min. Sample aliquots were generated at each step and analyzed by LC−MS. Reaction of Protein Persulfides with MMTS or BBMTS Followed by NACP. A freshly prepared protein persulfide solution (20 μM final concentration) was incubated with the methanethiosulfonate (2 mM final concentration) at rt for 30 min and then purified through a P-30 spin column. The sample solution was then incubated with NACP (2 mM final concentration) at rt for 3 h and purified as before. Sample aliquots were generated at each step and analyzed by LC−MS. ■ ASSOCIATED CONTENT S Supporting Information * Synthesis/preparation of the materials; LC−MS analysis; NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org. ■ AUTHOR INFORMATION Corresponding Author *(K.S.C.) E-mail: kcarroll@scripps.edu. Phone: (561) 2282460. Fax: (561) 228-2919. Notes The authors declare no competing financial interest. ■ ■ ACKNOWLEDGMENTS This work was supported by the National Institutes of Health (Grant No. GM102187). REFERENCES (1) Walsh, C. T., Garneau-Tsodikova, S., and Gatto, G. J. (2005) Protein post transilational modifications: the chemistry of proteome diversifications. Angew. Chem., Int. Ed. 44, 7342−7372. (2) Reddie, K. G., and Carroll, K. S. (2008) Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 12, 746−754. (3) Mieyal, J. J., and Chock, P. B. (2012) Posttranslational modification of cysteine in redox signaling and oxidative stress: focus on S-glutathionylation. Antioxid. Redox Signaling 16, 471−475. (4) Mustafa, A. K., Gadalla, M. M., Sen, N., Kim, S., Mu, W., Gazi, S. K., Barrow, R. K., Yang, G., Wang, R., and Snyder, S. H. (2009) H2S signals through protein S-sulfhydration. Sci. Signal. 2, ra72. (5) Kabil, O., and Banerjee, R. (2010) Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 285, 21903−21907. (6) Krishnan, N., Fu, C., Pappin, D. J., and Tonks, N. K. (2011) H2Sinduced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 4 4, ra86. (7) Sen, N., Paul, B. D., Gadalla, M. M., Mustafa, A. K., Sen, T., Xu, R., Kim, S., and Synder, S. H. (2012) Hydrogen sulfide-linked G dx.doi.org/10.1021/cb4001052 | ACS Chem. Biol. XXXX, XXX, XXX−XXX Persulfide Reactivity in the Detection of Protein S-sulfhydration Jia Pan and Kate S. Carroll* Supporting information Synthesis/preparation of the materials ………………..…………………………………………………S2-S3 LC-MS analysis …………………………………………………………………………………………S4-S13 References………………………………………………………………………………..........................S14 NMR Spectrum …………………………………………….……………………………………………S15-S18 S1 Materials and Methods: Unless otherwise noted, all reactions were performed under an argon atmosphere in oven-dried glassware. All purchased materials were used without further purification. Thin layer chromatography (TLC) was carried out using Analtech Uniplate silica gel plates. Yields refer to chromatographically and spectroscopically pure compounds, unless otherwise stated. NMR spectra were obtained on a Varian Inova 400 (400 MHz for 1H; 100 MHz for 13 C). 1H and 13 C NMR chemical shifts are reported in parts per million (ppm) relative to chloroform (δ 7.26) for 1H NMR and chloroform (δ 77.0) for 13C NMR. Low-resolution electrospray ionization (ESI) mass spectra of small-molecules were analyzed by an Agilent 6120 Quadruple LC/MS spectrometer on Poroshell 120 (Agilent) or ZIC®-pHILIC HPLC column (the Nest group). Protein intact mass analysis was performed on a Thermo LTQ XL coupled with Agilent 1100 mass spectrometer. Synthesis of S-4-Bromobenzyl methanethiosulfonate (BBMTS) A solution of sodium methanethiosulfonate (134 mg, 1 mmol), 4-bromobenzylmercaptan (250 mg, 1 mmol) and sodium iodide (7.5 mg, 0.05 mmol) in methanol (7 mL) was stirred and refluxed for 12 hours. After the reaction was done, the solution was partitioned between ethylacetate and saturated ammonium chloride solution. The organic layer was washed with water and brine, separated and dried over anhydrous magnesium sulfate. After filtration and evaporation, the residue was purified through flash chromatography to give product as a white solid (101 mg, 35%). m.p. 76-77 ℃; 1H NMR (400 MHz, CDCl3): δ 7.42 (2H, d, J = 8.4 Hz), 7.21 (2H, d, J = 8.8 Hz), 4.25 (2H, s), 2.91 (3H, s); 13 C NMR (75 MHz, CDCl3): δ 134.2, 132.1, 130.7, 122.3, 51.2, 40.0; IR (thin film) cm-1 2924, 1590, 1487, 1404, 1315, 1129, 1070, 1011, 954, 819, 804, 743. Synthesis of N-acetylcysteine pyridyldisulfide (NACP) S2 To a stirred solution of pyridyldisulfide (280 mg, 1 mmol) in THF (3 mL) was added dropwise a solution of N-acetylcysteine (163 mg, 1 mmol) in THF (2 mL), and the solution was stirred at room temperature for 1 hour. After evaporation of the solvent, the residue was purified through flash chromatography to give product as a light yellow oil (156 mg, 57%). 1H NMR (400 MHz, MeOD-d4): δ 8.26 (1H, d, J = 4.0 Hz), 7.65 (2H, d, J = 2.4 Hz), 7.08 (1H, d, J = 3.2 Hz), 4.55 (1H, q, J = 4.0 Hz), 3.20 (1H, dd, J1 = 14.0 Hz, J2 = 4.0 Hz), 3.02 (1H, dd, J1 = 14.0 Hz, J2 = 8.4 Hz), 1.84 (3H, s); 13C NMR (75 MHz, MeOD-d4): δ 173.4, 160.8, 150.6, 139.3, 122.7, 121.6, 53.4, 41.6, 22.6; IR (thin film) cm-1 3258, 2061, 2926, 1723, 1643, 1574, 1561, 1447, 1417, 1375, 1292, 1223, 1118, 1043, 762, 717; MS m/z 271.0 [M-H+]. S3 Figure S1. Mass spectrum of the reaction between glutathione persulfide and and N-ethylmaleimide (* the mass in this peak could not be assigned to a chemical structure). Figure S2. Mass spectrum of the reaction between papain persulfide and N-ethylmaleimide. Figure S3. Mass spectrum of the reaction between Gpx3 persulfide and N-ethylmaleimide (*the mass in this peak could not be assigned to a chemical structure). S4 Due to the difficulty to measure the fast kinetics of the reactions between thiols and MMTS/BBMTS, a competitive reactivity study was carried out. Biologically relevant thiols - glutathione (GSH) and N-acetylcysteine (NAC) reacted with MMTS and BBMTS to give product ratios close to 1:1, which proved the similar reactivity of BBMTS to MMTS. Table S1. Competitive reaction of MMTS and BBMTS with glutathione (GSH). To a mixed solution of MMTS and BBMTS (500 µM final concentration each) was added GSH (20, 40, and 100 µM final concentration, respectively) or N-acetylcysteine (NAC) (50, 100, and 200 µM final concentration, respectively), and the solutions were stirred at room temperature for 30 minutes before taken into LC-MS analysis. The concentration of the products was calculated based on the standard curve of the UV absorption of the products versus the sulfhydryl concentration. Entry 1 2 3 GSH / uM 20 40 100 Compound 15 / uM 7.75 13.8 42.9 Compound 33 / uM 6.25 24.2 69.1 Compound 15 Compound 33 1 2 3 S5 Table S2. Competitive reaction of MMTS and BBMTS with N-acetylcysteine. To a mixed solution of MMTS and BBMTS (500 µM final concentration each) was added N-acetylcysteine (NAC) (50, 100, and 200 µM final concentration, respectively), and the solutions were stirred at room temperature for 30 minutes before taken into LC-MS analysis. The concentration of the products was calculated based on the standard curve of the UV absorption of the products versus the sulfhydryl concentration. Entry 1 2 3 GSH / uM 50 100 200 Compound 34 / uM * * 77 Compound 35 / uM * 34.4 85.3 Compound 34 Compound 35 1 2 3 * undetectable concentration due to the weak UV absorption or ionization capability of the N-acetylcysteine derivatives S6 Formation of thiosulfate 27. Thiosulfate 27 was proposed to be the oxidation product of 4-bromobenzyl persulfide 36, a byproduct that could be generated via different pathways in the reaction of GSSH with BBMTS. Figure S4. Proposed mechanism of thiosulfate 27 formation in the reaction between glutathione persulfide and BBMTS. Further treatment of BBMTS with Na2S also yielded thiosulfate 27, which suggests that the sulfane sulfur of the persulfide species (such as 36) is highly susceptible to aerial oxidation to form thiosulfate (oxidation of persulfide sulfane sulfur to sulfate in aerobic conditions has been previously reported) [2]. When Na2S was the limiting reagent, the sulfate concentration (from the sulfane sulfur in both 27 and 39) correlates linearly with the concentration of Na2S (figure S6). However, when excess Na2S was used, the sulfate concentration decreased with the increasing concentration of Na2S (figure S7). S7 140000 y = 347.52x -­‐ 12880 R² = 0.9971 120000 100000 80000 60000 40000 20000 0 0 Figure S5. 100 200 300 400 500 Linear correlation between the sulfate formation (27+39) and the concentration of Na2S (limiting reagent). 500 µL each of BBMTS (1 mM final concentration) in Tris (100 mM, pH 7.4) was mixed with Na2S (40 µM, 100 µM , 200 µM, 400 µM and 1 mM final concentration, respectively) at room temperature for 30 minutes. 30000 25000 20000 15000 10000 5000 0 0 Figure S6. 1000 2000 3000 4000 5000 Relationship between the sulfate formation (39) and the concentration of Na2S (in excess): 500 µL each of BBMTS (200 µM final concentration) in Tris (100 mM, pH 7.4) was mixed with Na2S (200 µM, 400 µM , 1 mM 2 mM and 5 mM final concentration, respectively) at room temperature for 30 minutes. To explain why thiosulfate 27 did not exist in the excess Na2S and the concentration of thiosulfate 39 decreased with increasing concentration of Na2S, we proposed that excess Na2S reduced both thiosulfate 27 and 39 back to S8 persulfide intermediate 40, which undergo oxidation to form only thiosulfate 39. Although thiosulfate 39 and persulfide 40 should be maintained to similar level under the equilibrium, the nucleophilic substitution from 39 to 40 must be more kinetically and thermodynamically significant than the aerial oxidation from 40 to 39, therefore led to a decreasing trend of thiosulfate 39 as well. Figure S7. Proposed mechanism of the reaction between BBMTS and Na2S. Freshly prepared Na2S solution (1 mM final concentration) and BBMTS (1 mM final concentration) were mixed for 10 minutes at room temperature, and the products were analyzed in LC-MS. S9 Persulfide has been reported to have anti-oxidant property in biological systems [3-4] .To clarify the oxidation pathways, various anti-oxidative conditions have been tested, and found to inhibit the thiosulfate formation, similar to a recent report by Gate, K. S. et al[5]. As shown in table S3, compared to the reaction condition without any additive, the thiosulfate formation was suppressed in the presence of radical scavenger MeOH and DMSO, metal chelator EDTA, and H2O2 decomposer catalase. Those data suggest that the sulfane sulfur of the persulfide intermediate may be susceptible to different oxidation pathways. In addition, persulfide was known to react with oxygen molecule to generate sulfate and thiosulfate anions.[6] All those evidence may also explain the thiosulfate formation in the protein persulfide experiments. Table S3. Thiosulfate formation in the presence of antioxidants. To a solution of BBMTS (1 mM final concentration) in 500 µL Tris (100 mM, pH 7.4) with conditions: a. MeOH (1 M); b. DMSO (1 M); c. EDTA (20 mM); d. catalase (100 U/mL); and e. no additive, was added Na2S (500 µM final concentration). After 30 minutes, the product formation was measured in LC-MS. No addi9ve MeOH (1 M) DMSO (1 M) EDTA (20 mM) catalase (100 U/mL) S10 Figure S8. Mass spectrum of the Gpx3 persulfide reaction with a NACP; b DTNB; c DTT; d GSH; e NAC; f sulfite; g methane sulfinate; h dimedone; i malononitrile; j methyl acetoacetate; k N,N’-dimethyl barbituric acid. S11 Reactivity of thiosulfate towards nucleophiles: Table S4. The reactivity of Fmoc-cysteine thiosulfate towards nucleophiles. S12 Figure S9. The reaction of Fmoc-cysteine thiosulfate with A. DTT; B. GSH; C. NAC; D. methyl sulfinate; E. thiourea; F. dimedone and G. malononitrile (* represent peaks with unidentified mass). S13 References [1] Francoleon, N. E.; Carrington, S. J.; Fukuto, J. M. (2011) The reaction of H2S with oxidized thiols: Generation of persulfides and implications to H2S biology. Arch. Biochem. Biophys. 516, 146. [2] Everett, S. A.; Schoneich, C.; Stewart, J. H.; Asmus, K. (1992) Perthiyl radicals, trisulfide radical ions, and sulfate formation. A combined photolysis and radiolysis study on redox processes with organic di- and trisulfides. J. Phys. Chem. 96, 306. [3] Everett, S. A.; Folkers, L. K.; Wardman, P. (1994) Free-radical repair by a novel perthiol: reversible hydrogen transfer and perthiyl radical formation. Free Rad. Res. 20, 387. [4] Everett, S. A.; Wardman, P. (1995) Perthiols as antioxidants: radical-scavenging and prooxidative mechanisms. Methods in Enzymology 251, 55-69. [5] Chatterji, T.; Keerthi, K.; Gates, K. S. (2005) Generation of reactive oxygen species by a persulfide (BnSSH). Bioorg. Med. Chem. Lett. 15, 3921-3924. [6] Kabil, O.; Banerjee, R. (2012) Characterization of patient mutations in human persulfide dioxygenase (ETHE1) involved in H2S catabolism. J. Biol. Chem. 287, 44561. S14 S15 S16 S17 S18