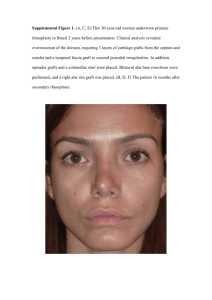
Isoenzyme Activities Differ in Compatible L. No.
advertisement

Forest Sci., Vol. 24, No. 2, 1978, pp. 297-303 Isoenzyme Activities Differ in Compatible and Incompatible Douglas..fir Graft Unions DONALD L. COPES ABSTRACT. Unusually dark-stained peroxidase bands at Rm (relative mobility) 0.77 to 0. 80 and esterase bands at Rm 0.50 to 0. 56 were found in tissues of incompatible Douglas­ fir graft unions. Ninety- to 100-percent accuracy in identifying incompatible grafts was achieved with union tissues 10 to 15 months after grafting (February to May). A practical graft testing program is possible using electrophoretic methods for detecting changes in stain intensity in bands from incompatible graft unions. Enzyme activity of bands with other Rm values was not correlated, or was only weakly correlated, with graft incompati­ bility. Enzyme similarity of stock and scion tissues could not be used to determine poten­ tial compatibility of stock-scion combinations before grafting. No correlation with graft incompatibility was found with enzyme activity or the presence or absence of isoenzyme bands of catalase, acid phosphatase, or leucine aminopeptidase. ADDITIONAL KEY WORDS. FoREST Scr. 24:297-303. Vegetative propagation, Pseudotsuga menziesii. PLANT TISSUES formed in graft unions are composed of cells similar to those found in areas previously subjected to wounding or injury. New graft unions in conifers are typically composed of irregularly shaped parenchyma cells (callus) and irregu­ larly shaped and oriented tracheids and phloem elements. Although the cell types in unions and wounds are similar, one condition present in unions is very different from that found in wounds-the contiguous contact of cells of unlike genotypes. Altered starch metabolism patterns have already been detected in graft tissues (Mel­ nick and others 1964), but studies of enzyme activity in graft unions of conifers have not been reported. A good test organism for enzymatic study of graft unions should exhibit a high degree of cellular antagonism when genetically dissimilar individuals are grafted. Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) meets this criterion-more than one-third of the grafts made with Douglas-fir stocks and scions of unlike geno­ types soon show visible signs of between-cell antagonism. The first visible symptom is the development of suberized zones between some cells of the stock and scion 3 to 4 months after grafting (Copes 1970). Necrotic areas develop later in and around the suberized areas in about 35 percent of the unions. These grafted trees ultimately die of bark girdles and are said to have been incompatible grafts. The necrotic areas closely resemble the zones of controlled necrosis, described by Berryman ( 1972). Such zones occurred in plants as a result of the "hypersensitive reaction" mecha­ nism. In many cases, hypersensitive reactions are initiated as defense reactions to pathogen attack. Graft-induced between-cell antagonism in Douglas-fir may result from activation of its hypersensitive reaction mechanism. The incompatible reaction may be triggered by the ability of contiguous cells of unlike genotypes to recognize The author is principal plant geneticist, Forestry Sciences Laboratory, Pacific Northwest Forest and Range Experiment Station, USDA Forest Service, Corvallis, Oregon. Manuscript received 13 October 1977. VOLUME 24, NUMBER 2, 1978 I 297 the adjacent Douglas-fir cells as foreign bodies and, thus, to react to prevent inva­ sion. Anatomical evidence supports this hypothesis (Copes 1970). Activation of hypersensitive systems has been correlated with elevated activity of some oxidative enzymes and increased synthesis of phenolic compounds (Berryman 1972). If antagonism in Douglas-fir unions is caused by a hypersensitive reaction system, then the peroxidase, or polyphenol oxidase, enzyme systems should be examined. Legrand and others (1976) found the phenylpropanoid pathway directly linked to hypersensitive necrosis reactions in tobacco plants. High levels of phenolic compounds in the stocks and scions of cherry trees were correlated with graft failure ( Yu 1972). Peroxidases exert direct control over the level of polyphenols in plants by the catabolism of phenylpropanoid compounds (Konarev 1972). Indirect evidence for a role of oxidative enzymes upon intercellular antagonism was seen in bean plants where high peroxidase levels precede leaf abscission (Poo­ vaiah and Rasmussen 1973). Protective layers of cells in abscission zones closely resemble graft rejection zones found in incompatible Douglas-fir unions. Peroxi­ dase activity correlates with the level of endogenous indole-3-acetic acid (Galston and Davies 1969) and interacts with cytokinins and gibberellins (Lee 1972)­ which is further evidence that peroxidase enzymes are good monitors of physiologi­ cal responses. In the present Douglas-fir study, isoenzymes of peroxidase, catalase, leucine aminopeptidase, acid phosphatase, and esterase were studied in bark tissues of unions of known compatible and incompatible stock-scion combinations, in wounds, and in nonunion areas of stock and scions. Tissues were sampled at 17 different graft ages over a 3-year period. Consistent differences between the enzymes from compatible and incompatible grafts support the hypothesis that hypersensitive reac­ tions were responsible for between-cell antagonism in Douglas-fir graft unions. METHODS Douglas-fir grafts were made on 11- and 12-year-old stock trees during April 1973 and April 1974. Three trees (S-1, S-2, LW-2) 20 to 30 years old were selected as sources of scions for homoplastic grafts (grafts with scion and stocks of the same species but of unlike genotypes). Grafts were placed on branch tips of eight stock trees in 1973 and on five different stock trees in 1974. At least 20 grafts of each scion-clone were made on each rootstock. Twenty autoplastic grafts (grafts with scion and stock of identical genotypes) of each of the five rootstocks were made in 1974, but none were grafted in 1973. Plant collections for electrophoresis were made 1 to 4 days prior to electropho­ resis. Plant material was stored in plastic bags at about 2 °C until used. One graft of each scion-stock combination was examined in each electrophoretic run. Grafts were examined from 1 to 27 months after grafting. Twenty-one electrophoresis runs were made from May 1973 to October 1975. Samples of bark included all tissues from the periderm to inner cambium. Two samples were cut from nongrafted areas of the scion and stock (2.5 em above and 5.0 em below the graft union). Another sample was cut from a wound area near the union but not in the area where stock and scion tissues merged. The fourth sample was cut from the narrow union zone where the stock and scion cells grew together. The latter sample was a 2- to 3- by 10-mm piece of bark which was cut from the graft with a surgical scalpel. The exact perimeter of the union zone could easily be identified through the first two growing seasons but became increasingly difficult during the third growing season. For half the trees an additional sample was made by mixing equal weights of tissue from nonunion areas of both scion and stock. This sample was made to determine if changes in bands could occur if two different 298 I FOREST SCIENCE genotypes were mixed in vitro vs. the in vivo condition found in unions. The com­ posite sample was substituted for wound samples. For all samples, 100- to 250-mg of tissue was macerated just prior to the start of each electrophoretic run in 200 to 500 p.l of gel buffer solution containing 4.5 percent polyvinylpyrrolidone (MW 40,000). A ratio of 100 mg of tissue to 200 p.l of gel buffer was maintained. Fluid extracts from the macerated tissues were absorbed on 5- by 13-mm paper wicks cut from Whatman No. 1 chromatography paper and immediately inserted into the starch gels at the origin slit. Four sample wicks from each graft (one each from the stock, union, scion, and combined scion-stock or wound) were inserted side by side in the same gel. Starch gel electrophoresis generally followed the methods outlined by Conkle (1972) and modified by Copes (1975). Gels (12 percent and pH 8.0) were poured into molds 20- by 13- by 1.6-cm. Each gel contained 461 ml of gel buffer solution (417 ml tris-citrate [0.5 M tris and 0. 1 M citric acid, pH 8.3] and 50 ml lithium borate [0.03 M lithium hydroxide and 0. 17 M boric oxide, pH 7.4]). Lithium borate (0. 03 M lithium hydroxide and 0.17 M boric oxide) was used as the electrode buffer solution. Constant current of 75 rna was applied until a bromo­ phenol blue marker dye had moved 8 em from the origin slit. Voltage averaged about 400 V (220-540 V) and gel temperatures averaged 8°C (5°- 14°C) during an average 4- to 4.5-hour run. After electrophoresis, each gel was sliced horizontally and different slices of each gel were stained for peroxidase, catalase, leucine aminopeptidase, esterase, and acid phosphatase. The esterase, acid phosphatase, and leucine aminopeptidase were stained by procedures described by Scandalios (1969). Peroxidase (polyphenol oxidase) was tested with three different hydrogen donors: benzidine (Scandalios 1969), 3, 3-dimethoxy-benzidine (Brewbaker and others 1968), and N, N-dimethyl­ p-phenylenediamine (Molnar and LaCroix 1972). Catalase was tested by the Shaw and Prasad ( 1970) recipe for bacteria. Bands from isoenzymes were recorded in photographs and diagrams. Stain inten­ sity was recorded in addition to relative migration distance. Bowling and Crowden ( 1973) found a good correlation between peroxidase stain intensity in gels and that obtained by regular assay methods on soluble peroxidases; thus, major increases or decreases in intensity of staining of individual isoenzymes were used in the present study to indicate possible quantitative differences in enzyme activity. Because of the inexactness of this method, only isoenzymes with pronounced visible differences were recorded as having significantly increased or decreased activity. Within-tree comparisons were made between tissues from union and nonunion areas, as well as between-tree comparisons of tissues taken from compatible and incompatible unions. Bands were measured to the closest mm and transformed into relative mobility units (Rm) : the distance the isoenzyme traveled from the origin slit toward the anode, divided by the distance a bromophenol blue marker dye had moved from the origin slit toward the anode. The part of each graft union that remained after electrophoresis analysis was preserved in 50-percent ethanol for later anatomical study. The preserved graft unions were cross-sectioned, stained, and microscopically examined to determine graft compatibility (Copes 1970). These anatomical data were then correlated with the isoenzyme data to determine which isoenzymes might be useful to detect incompatible or compatible grafts. RESULTS Anatomical tests of unions of homoplastic grafts showed 50 percent in 1973 and 53 percent in 1974 to be incompatible. No incompatibility symptoms were detected in VoLUME 24, NuMBER 2, 1978 1 299 TABLE Percent accuracy achieved in correctly identifying compatible and 1. incompatible graft unions of increasing age with electrophoresis methods. Unions correctly identified 1973 grafts• Peroxidase Month Graft age Month s 1974 grafts• Peroxidase Esterase, Esterase, Rm0.27 Rm0.44 Rm0.77 Rm0.50 Rm0.27 Rm0.44 Rm0.77 Rm0.50 and0.35 and0.49 and0.80 and0.56 and0.35 and0.49 and0.80 and0.56 . _______ --_____________ . _____________________ _c -Percent_____------__________________________---______ May 1 0 0 0 June 2 0 0 0 0 July 3 0 0 0 65 August 4 0 63 0 60 September 5 0 85 0 65 November 7 0 0 0 0 0 0 0 65 0 0 100 96 0 0 100 100 0 0 93 90 0 0 100 85 0 0 95 90 February 10 April 12 May 13 July August October 18 0 0 77 76 November 19 0 0 79 71 0 0 94 15 0 0 96 92 16 71 0 92 67 96 January 21 0 0 87 62 February 22 83 55 83 75 April 24 0 75 83 79 July 27 0 0 87 65 • 0 = 24 graft unions sampled each month. • = 20 graft unions sampled each month. c = not sampled. autoplastic grafts. Two scion clones (LW-2 and S-2) were both compatible or both incompatible when grafted on the same stocks, but the third scion clone (S- 1) always had the opposite compatibility of LW-2 and S-2. Similarity of nonunion scion and stock tissues had no predictive value in deter­ mining which combinations would form compatible graft unions. Scion clones and stock trees which formed compatible graft unions were no more similar than scions and stocks which formed incompatible combinations. The presence or absence of particular bands had no relationship to whether a graft union would be compatible or incompatible. Noticeable differences in enzyme activity were evident in comparisons of tissues from compatible and incompatible unions. Certain bands from tissues of incom­ patible unions stained much darker than the same bands from tissues of compatible unions or from nonunion areas. These unusually dark-stained bands occurred at Rm 0.44, 0.49, 0.77, and 0.80 for peroxidase and Rm 0.50 and 0.56 for esterase. The percentage of trees correctly identified at different graft ages as having incom­ patible graft unions is shown in Table 1. Correct identification was made when electrophoretic results corresponded with anatomical test results. Incompatible grafts could be accurately identified by electrophoretic techniques only during specific months or at certain graft ages. At other times, high general activity in all union tissues made detection of incompatible-correlated increases dif­ ficult. Increases in enzyme activity in union tissues at Rm 0. 77 and Rm 0.80 were not correlated with graft incompatibility until February, 10 months after grafting. 300 I FOREST SCIENCE A B FIGURE 1. C 0 A 8 C 0 A B C D A B C 0 The two arrows point to location of Rm 0.44 and 0.49 peroxidase bands from tissues of two incompatible graft unions. The other bands were from two compatible unions and from nonunion areas of the scion and stock (A, stock; 8, union; C, scion; D, scion + stock). At that time, 100 percent of the incompatible grafts were identified with either peroxidase or esterase bands. Between 90- and 1 00-percent accuracy in detecting incompatible grafts was achieved from that time until the following May for both the Rm 0.77 and 0.80 peroxidase bands and the Rm 0.50 and 0.56 esterase bands, and until August if only the peroxidase bands were used. After that time, the detection accuracy obtained with peroxidase and esterase averaged 84 percent and 74 percent for the remaining 12 months of the study (Table 1). A general increase in activity of Rm 0.44 and 0.49 peroxidase was noted in wounds and in both autoplastic and homoplastic graft tissues. This increase may have resulted from the numerous callus or parenchyma cells in wound and graft tissues, and was not correlated with graft incompatibility. An even darker staining of these same bands was noted 5 months after grafting. At that time 85 percent of the incompatible unions could be identified by the unusually dark-stained Rm 0.44 and 0.49 peroxidase bands (Fig. 1, Table 1). These bands also showed correlation with incompatibility 4 and 22 months after grafting. Bands other than the Rm 0.50 and 0.56 esterase and the Rm 0.44, 0.49, 0.77, and 0.80 peroxidase from union tissues occasionally stained a darker color than bands with the same relative mobility from nonunion tissues, but their presence had little or no correlation with graft incompatibility. Such bands were found at Rm 0.27, 0.35, 0.40, 0.66, and 0.71 for peroxidase and at Rm 0.37, 0.69, 0.75, 0.80, 0.83, and 0.87 for esterase. DISCUSSION Incompatible graft unions were identified most accurately by electrophoresis 10 to 15 months after grafting. At that time, 90 to 100 percent of the incompatible unions were detected using the Rm 0.50 and 0.56 esterase and the Rm 0.77 and 0.80 peroxidase bands. Detection accuracy decreased after the 15th month because tissues cut from older grafts contained too much nonunion scion or stock tissue. A program to detect incompatible stock-scion combinations by electrophoretic tech­ niques is possible if union tissues of the proper age are tested, but similarity of iso­ enzymes of stock trees and scion clones is useless in predicting compatibility prior to grafting. Electrophoretic detection of incompatible grafts has several advantages over the anatomical test method (Copes 1970) currently used. First, the unions need not be destroyed since only a thin slice of bark from the area where the stock and scion tissues join is reguired. Thus, only one graft is needed on each stock. A second advantage is that reliable electrophoretic testing can be done 10 months after graft­ ing, whereas reliable anatomical testing is not possible until the grafts are at least 16 to 17 months old. VOLUME 24, NUMBER 2, 1978 I 301 The actual cause of between-cell antagonism was not found, but the increase in peroxidase activity suggests that the hypersensitive reaction mechanism had been activated. Reliable electrophoretic identification of incompatible graft unions was not possible until after visible symptoms of between-cell antagonism were seen through a microscope. Minor isoenzyme differences, detected from 3 to 7 months after grafting, correlated somewhat with the appearance of suberized and necrotic tissues in graft unions of incompatible combinations (Copes 1970). The character­ istic darker staining of peroxidase bands from tissues in incompatible graft unions may have been associated with cell necrosis. A similar role of phenols and cell necrosis was demonstrated in hypersensitive reactions of tobacco plants (Legrand and others 1976). Increases in esterase activity have been reported in spruce needles and birch leaves injured by fluoride emissions (Yee-Meiler 1975). The altered esterase bands found in this study may also be caused from cellular antago­ nism (necrosis). Comparisons of zymograms from union and nonunion tissues graphically demon­ strate union and wound enzyme activities to be different than enzyme activity seen in nongrafted or healthy tissues. The most significant difference was darkly stain­ ing bands at Rm 0.27, 0.35, 0.44, and 0.49, indicating an increase in peroxidase activity in practically all graft unions and wound areas. No isoenzyme differences between compatible and incompatible grafts could be detected with leucine aminopeptidase, acid phosphatase, or catalase. LITERATURE CITED BERRYMAN, A. A. 1972. Resistance of conifers to invasion by bark beetle-fungus associations. Bioi Sci 22:59 8-602. BowLING, A. C., and R. K. CROWDEN. 1973. Peroxidase activity and lignification in the pod membrane of Pisum sativum L. Aust J Bioi Sci 26:679-684. BREWBAKER, J.L., M. D. UPADHYA, Y. MAKINEN, and T. MACDONALD. 1968. Isoenzyme poly­ morphism in flowering plants. III. Gel electrophoresis methods and applications. Physiol Plant 21:930-940. CoNKLE, M. T. 1972. Analyzing genetic diversity in conifers ... isoenzyme resolution by starch gel electrophoresis. USDA Forest Serv Res Note PSW-264, 5 p. Pac Southwest Forest and Range Exp Stn, Berkeley, Calif. CoP Es, D. 1970. Initiation and development of graft incompatibility symptoms in Douglas­ fir. Silvae Genet 19:10 1-107. CoPEs, D. L. 1975. Isoenzyme study of dwarf and normal Douglas-fir trees. Bot Gaz 136: 347-352. GALSTON, A.W., and P. J. DAVIES. 1969. Hormonal regulation in higher plants. Science 163:1 2 8 8- 1297. KoNAREV, V. G. 1972. Cytochemistry and histochemistry of plants (Tsitokhimiya i gisto­ khimiya rastenii). Translated by P. Harry. LEE, T. T. 1972. Jerusalem, Israel Program Sci Trans!, 260 p. Interaction of cytokinin, auxin and gibberellin on peroxidase isoenzymes in tobacco tissues cultured in vitro. Can J Bot 50:2471-2477. LEGRAND, M. , B. FRITIG, and L. HmTH. 1976. Enzymes of the phenylpropanoid pathway and the necrotic reaction of hypersensitive tobacco to tobacco mosaic virus. Phytochemis­ try 15:1353-1359. MELNICK, V. L., L. HOLM, and B. E. STRUCKMEYER. 1954. Physiological studies on fruit development by means of ovule transplantation in vivo. Science 145:609-61 1. MoLNAR, J. M., and L. J.LACROIX. 1972. macrophyl/a: Studies of the rooting of cuttings of Hydrangea enzyme changes. Can J Bot 40:31 5-322. PooVAIAH, B. W., and H. P. RAsMUSSEN. 1973. Peroxidase activity in the abscission zone of bean leaves during abscission. Plant Physiol 52:263-267. SCANDALJOS, J. G. 1969. Genetic control of multiple molecular forms of enzymes in plants: A review. Biochem Genet 3:37-79. 302 I FOREST SCIENCE SHAW, C. R., and R.PRASAD. 1970. Starch gel electrophoresis of enzymes-a compilation of recipes. Biochem Genet 4:297-320. YEE-MEILER, D. 1975. On the suitability of phosphatase and esterase activity in spruce needles and birch leaves for detecting invisible physiological damage by fluoride emissions. Eur J For Pathol 5:329-338. Yu, K. S. Comparative study of phenolic compounds in cherry rootstocks. Diss Abstr 1972. Int 32:6769-B. Forest Sci., Vol. 24, No. 2, 1978, pp. 303-308 Aspen Sucker Regeneration Following Burning and Clearcutting on Two Sites in the Rocky Mountains Note by George ABSTRACT. A. Schier and Robert B. Campbell Aspen (Populus tremuloides Michx.) root suckers arising after a control burn in Wyoming and clearcutting in Utah were studied to obtain information on depth and diameter of parent roots producing suckers, occurrence of new roots, and the effects of burn intensity on suckering. Compared with parent roots of aspen in the Lake States, those of western aspen were deeper and slightly larger. Clonal differences were found in the depth and diameter of parent roots and in the ability to initiate new roots around the base of suckers. Few suckers had well-developed independent root systems. A high burn inten­ sity increased the depth at which suckers were initiated. FoREST SCI. 24:303-308. ADDITIONAL KEY WORDS. Populus tremuloides, vegetative propagation, adventitious shoots, roots. FOLLOWING LOGGING OR FIRE, aspen (Populus tremuloides Michx.) reproduces vegeta­ tively from suckers (adventitious shoots) that develop from long, ropelike lateral roots. Suckers occur on parts of the undulating roots that rise near the soil surface (Baker 1925, Day 1944, Sandberg 19511). The only quantitative data describing the depth and diameter of roots that give rise to suckers are from the Lake States (Sandberg 1951, Farmer 1962). This information may not adequately describe regeneration in western aspen because of differences between regions in climate and soil and aspen genotypes. Therefore, when mature aspen was clearcut in one area of the Rocky Mountains and control burned in another as part of a research program on the ecology and management of aspen, we collected data on depth and diameter of parent roots of aspen suckers. We also studied the formation of new roots and the effect of burn intensity on aspen regeneration. 1 Sandberg, D. 1951. Univ Minn. The regeneration of quaking aspen by root suckering. M. F. Thesis, 172 p. The authors are, respectively, Plant Physiologist and Biological Technician, Intermountain Forest and Range Experiment Station, Forest Service, U.S. Department of Agriculture, Ogden, Utah 84401, located at the Intermountain Station's Forestry Sciences Laboratory, Logan, Utah. Manuscript received 15 July 1977. VOLUME 24, NUMBER 2, 1978 I 303