Introduction to Amines
advertisement

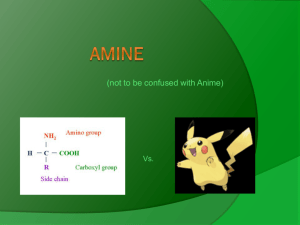
Introduction to Amines Amines are organic derivatives of ammonia. Amines, like ammonia, are weak bases (Kb = 10−4 to 10−6). This basicity is due to the unshared electron pair on the nitrogen atom. Amines occur widely in both plants and animals tissues .Nicotine is found in tobacco ;and cocaine is found in the south American coca bush ,Amino acids are the building block from all protein are made and cyclic amine basis are constituents of nucleic acid. Classification and nomenclature of amines Amines are classified as primary, secondary, or tertiary based upon the number of carbon-containing groups that are attached to the nitrogen atom. Those amine compounds that have only one group attached to the nitrogen atom are primary, while those with two or three groups attached to the nitrogen atom are secondary and tertiary, respectively. Amines named Aliphatic amines are named by naming the alkyl group or groups attached t» nitrogen, and following these by the word -amine. More complicated ones are often named by prefixing amino- (or N-methylamino-y N,N-diethylamino-, etc.) to the name of the parent chain. For example: Aromatic amines—those in which nitrogen is attached directly to an aromatic ring—are generally named as derivatives of the simplest aromatic amine, aniline. An aminotoluene is given the special name of toluidine. For example: Salts of amines are named by replacing –amine by ammonium (or –aniline by anilinium ) and adding the name of the anion (chloird ,nitrate,,sulfate,etc.)for example Heterocyclic amines—compounds in which the nitrogen atom i of a ring—are also common, and each different heterocyclic ring! its own parent name. The heterocyclic nitrogen atom is always position. Physical properties of amine Like ammonia, amines are polar compounds and, except for tertiary amines, :an form intermolecular hydrogen bonds. Amines have higher boiling points than non-polar compounds of the same molecular weight, but lower boiling points ban alcohols or carboxylic acids. Amines of all three classes are capable of forming hydrogen bonds with water. Vs a result, smaller amines are quite soluble in water, with borderline solubility being reached at about six carbon atoms. Amines are soluble in less polar solvents like ether alcohols ,benzene.Aromatic amines are very toxic. They are readily absorbed though the skin, often with fatal results. Basicity of amines Amines are basic because they possess a pair of unshared electrons, which they can share with other atoms. These unshared electrons create an electron density around the nitrogen atom. The greater the electron density, the more basic the molecule. Groups that donate or supply electrons will increase the basicity of amines while groups that decrease the electron density around the nitrogen decrease the basicity of the molecule. For alkyl halides in the gas phase, the order of base strength is given below: (CH3)3 N > (CH3)2NH > CH3NH2 > NH3 However, in aqueous solutions, the order of basicity changes. (CH3)2 NH > CH3NH2 > (CH3)3N > NH3 The differences in the basicity order in the gas phase and aqueous solutions are the result of solvation effects. Amines in water solution exist as ammonium ions. In water, the ammonium salts of primary and secondary amines undergo solvation effects (due to hydrogen bonding) to a much greater degree than ammonium salts of tertiary amines. These solvation effects increase the electron density on the amine nitrogen to a greater degree than the inductive effect of alkyl groups. Arylamines are weaker bases than cyclohexylamines because of resonance. Aniline, a typical arylamine, exhibits the resonance structures shown in Figure 1 . Preparation Of Amines Reactions of Amines Basicity. Salt formation. Uses of amines As drugs Chlorpheniramine is an antihistamine the helps to relief allergic disorders due to cold, hay fever, itchy skin, insect bites and stings. Diphenhydramine is the common antihistamine, benadryl. Chlorpromazine is a tranquillizer that sedates without inducing sleep. It is used to relieve anxiety, excitement, restlessness or even mental disorder. Acetaminophen is also known as paracetamol or p-acetaminophenol, an analgesic that relieves pains such as headaches. It is believed to be less corrosive to the stomach and is an alternative to aspirin.