1 ecture L Spectroscopy
advertisement

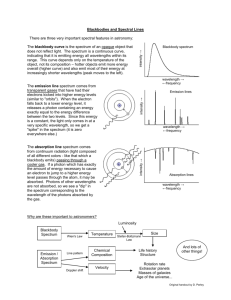
Lecture 1 Spectroscopy • It is the branch of science that deals with the study of interaction of electromagnetic radiation with matter. Electromagnetic Radiation • Electromagnetic radiation consist of discrete packages of energy which are called as photons. • A photon consists of an oscillating electric field (E) & an oscillating magnetic field (M) which are perpendicular to each other. Electromagnetic Radiation • Frequency (ν): – It is defined as the number of times electrical field radiation oscillates in one second. – The unit for frequency is Hertz (Hz). 1 Hz = 1 cycle per second • Wavelength (λ): – It is the distance between two nearest parts of the wave in the same phase i.e. distance between two nearest crest or troughs. Electromagnetic Radiation • The relationship between wavelength & frequency can be written as: c=νλ • As photon is subjected to energy, so E=hν=hc/λ Visible light: • The visible spectrum is the electromagnetic spectrum that is visible to the human eye. • The longest wavelength is red and the shortest is violet. Electromagnetic Radiation When visible light is passed through a prism, it split up into 7 coloures which correspond to definite wavelengths. This phenomenon is called dispersion. Violet 400 - 420 nm Yellow 570 - 585 nm Indigo 420 - 440 nm Orange 585 - 620 nm Blue 440 - 490 nm Red 620 - 780 nm Green 490 - 570 nm Electromagnetic spectrum: • The visible spectrum constitutes but a small part of the total radiation spectrum. Most of the radiation that surrounds us cannot be seen, but can be detected by dedicated sensing instruments. This electromagnetic spectrum ranges from very short wavelengths (including gamma and x-rays) to very long wavelengths (including microwaves and broadcast radio waves). The following chart displays many of the important regions of this spectrum. Electromagnetic Radiation Principles of Spectroscopy • The principle is based on the measurement of spectrum of a sample containing atoms / molecules. • Spectrum is a graph of intensity of absorbed or emitted radiation by sample verses frequency (ν) or wavelength (λ). • Spectrometer is an instrument design to measure the spectrum of a compound. Principles of Spectroscopy 1. Absorption Spectroscopy: • An analytical technique which concerns with the measurement of absorption of electromagnetic radiation. • e.g. UV (185 - 400 nm) / Visible (400 - 800 nm) Spectroscopy, IR Spectroscopy (0.76 - 15 μm) Principles of Spectroscopy 2. Emission Spectroscopy: • An analytical technique in which emission (of a particle or radiation) is dispersed according to some property of the emission & the amount of dispersion is measured. • e.g. Mass Spectroscopy UV-Visible Spectroscopy • A diagram of the components of a typical spectrometer are shown in the following diagram. The functioning of this instrument is relatively straightforward. A beam of light from a visible and/or UV light source (colored red) is separated into its component wavelengths by a prism or diffraction grating. Each monochromatic (single wavelength) beam in turn is split into two equal intensity beams by a half-mirrored device. One beam, the sample beam (colored magenta), passes through a small transparent container (cuvette) containing a solution of the compound being studied in a transparent solvent. The other beam, the reference (colored blue), passes through an identical cuvette containing only the solvent. The intensities of these light beams are then measured by electronic detectors and compared. The intensity of the reference beam, which should have suffered little or no light absorption, is defined as I0. The intensity of the sample beam is defined as I. Over a short period of time, the spectrometer automatically scans all the component wavelengths in the manner described. The ultraviolet (UV) region scanned is normally from 200 to 400 nm, and the visible portion is from 400 to 800 nm. • If the sample compound does not absorb light of of a given wavelength, I = I0. However, if the sample compound absorbs light then I is less than I0, and this difference may be plotted on a graph versus wavelength, as shown on the right. Absorption may be presented as transmittance (T = I/I0) or absorbance (A= log I0/I). If no absorption has occurred, T = 1.0 and A= 0. Most spectrometers display absorbance on the vertical axis, and the commonly observed range is from 0 (100% transmittance) to 2 (1% transmittance). The wavelength of maximum absorbance is a characteristic value, designated as λmax. Different compounds may have very different absorption maxima and absorbances. Intensely absorbing compounds must be examined in dilute solution, so that significant light energy is received by the detector, and this requires the use of completely transparent (non-absorbing) solvents. The most commonly used solvents are water, ethanol, hexane and cyclohexane. Solvents having double or triple bonds, or heavy atoms (e.g. S, Br & I) are generally avoided. Because the absorbance of a sample will be proportional to its molar concentration in the sample cuvette, a corrected absorption value known as the molar absorptivity is used when comparing the spectra of different compounds. This is defined as: • Molar Absorptivity,ε = A/ c l ( where A= absorbance, c = sample concentration in moles/liter & l = length of light path through the cuvette in cm.) • For the spectrum on the right, a solution of 0.249 mg of the unsaturated aldehyde in 95% ethanol (1.42 • 10-5 M) was placed in a 1 cm cuvette for measurement. Using the above formula, ε = 36,600 for the 395 nm peak, and 14,000 for the 255 nm peak. Note that the absorption extends into the visible region of the spectrum, so it is not surprising that this compound is orange colored. Molar absorptivities may be very large for strongly absorbing compounds (ε >10,000) and very small if absorption is weak (ε = 10 to 100). UV-Visible Absorption Spectra • To understand why some compounds are colored and others are not, and to determine the relationship of conjugation to color, we must make accurate measurements of light absorption at different wavelengths in and near the visible part of the spectrum. Commercial optical spectrometers enable such experiments to be conducted with ease, and usually survey both the near ultraviolet and visible portions of the spectrum. The visible region of the spectrum comprises photon energies of 36 to 72 kcal/mole, and the near ultraviolet region, out to 200 nm, extends this energy range to 143 kcal/mole. Ultraviolet radiation having wavelengths less than 200 nm is difficult to handle, and is seldom used as a routine tool for structural analysis. The energies noted above are sufficient to promote or excite a molecular electron to a higher energy orbital. • Consequently, absorption spectroscopy carried out in this region is sometimes called "electronic spectroscopy". A diagram showing the various kinds of electronic excitation that may occur in organic molecules is shown on the left. Of the six transitions outlined, only the two lowest energy ones (left-most, colored blue) are achieved by the energies available in the 200 to 800 nm spectrum. As a rule, energetically favored electron promotion will be from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO), and the resulting species is called an excited state. TYPES OF ELECTRONIC TRANSITIONS FOUR TYPES OF ELECTRONIC TRANSITIONS 1 σ→σ* for an ordinary carbon-carbon bond. (125-150)nm 2 π→π* for an isolated double bond. (160-190)nm 3 compounds. Aldehydes and ketones(275-295)nm n→π* In carbonyl 4 n→σ* in oxygen,nitrogen ,sulphur and Halogen compounds. (150-250)nm 21 When sample molecules are exposed to light having an energy that matches a possible electronic transition within the molecule, some of the light energy will be absorbed as the electron is promoted to a higher energy orbital. An optical spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. The resulting spectrum is presented as a graph of absorbance (A) versus wavelength, as in the isoprene spectrum shown below. Since isoprene is colorless, it does not absorb in the visible part of the spectrum and this region is not displayed on the graph. Absorbance usually ranges from 0 (no absorption) to 2 (99% absorption), and is precisely defined in context with spectrometer operation. Because the absorbance of a sample will be proportional to the number of absorbing molecules in the spectrometer light beam (e.g. their molar concentration in the sample tube), it is necessary to correct the absorbance value for this and other operational factors if the spectra of different compounds are to be compared in a meaningful way. The corrected absorption value is called "molar absorptivity", and is particularly useful when comparing the spectra of different compounds and determining the relative strength of light absorbing functions (chromophores). The functional groups containing multiple bonds capable of absorbing radiations above 200 nm due to n → π* & π → π* transitions. e.g. NO2, N=O, C=O, C=N, C≡N, C=C, C=S, etc • If the isoprene spectrum on the right was obtained from a dilute hexane solution (c = 4 * 10-5 moles per liter) in a 1 cm sample cuvette, a simple calculation using the above formula indicates a molar absorptivity of 20,000 at the maximum absorption wavelength. Indeed the entire vertical absorbance scale may be changed to a molar absorptivity scale once this information about the sample is in hand. Clicking on the spectrum will display this change in units. From the chart below it should be clear that the only molecular moieties likely to absorb light in the 200 to 800 nm region are pi-electron functions and hetero atoms having nonbonding valence-shell electron pairs. Such light absorbing groups are referred to as chromophores. A list of some simple chromophores and their light absorption characteristics is provided on the left above. The oxygen non-bonding electrons in alcohols and ethers do not give rise to absorption above 160 nm. Consequently, pure alcohol and ether solvents may be used for spectroscopic studies. • The presence of chromophores in a molecule is best documented by UV-Visible spectroscopy, but the failure of most instruments to provide absorption data for wavelengths below 200 nm makes the detection of isolated chromophores problematic. Fortunately, conjugation generally moves the absorption maxima to longer wavelengths, as in the case of isoprene, so conjugation becomes the major structural feature identified by this technique. Molar absorptivities may be very large for strongly absorbing chromophores (>10,000) and very small if absorption is weak (10 to 100). The magnitude ofε reflects both the size of the chromophore and the probability that light of a given wavelength will be absorbed when it strikes the chromophore. Chromophore Example Excitation λmax, nm ε Solvent C=C Ethene π __> π* 171 15,000 hexane C≡C 1-Hexyne π __> π* 180 10,000 hexane C=O Ethanal n π __> π* __> π* 290 180 15 10,000 hexane hexane N=O Nitromethane n π __> π* π* 275 200 17 5,000 ethanol ethanol C-X X=Br X=I Methyl bromide Methyl Iodide n n __> σ* σ* 205 255 200 360 hexane hexane __> __> Applications • Qualitative & Quantitative Analysis: – It is used for characterizing aromatic compounds and conjugated olefins. – It can be used to find out molar concentration of the solute under study. • Detection of impurities: – It is one of the important method to detect impurities in organic solvents. • Detection of isomers are possible. • Determination of molecular weight using Beer’s law.