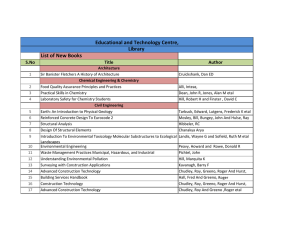
Saturn upper atmosphere structure D. Shemansky & X. Liu SET/PSSD

Saturn upper atmosphere structure
D. Shemansky & X. Liu
SET/PSSD
06/26/11
Occultation results
• The fuv spectrum exclusively provides hydrocarbon vertical structure.
• The euv spectrum exclusively obtains the properties of H
2 and HI. At the altitude of the hydrocarbon homopause the H
2 opacity removes measureable flux from the stellar or solar source.
10
-2
8
6
4
3
2
10
-3
8
6
4
3
2
10
-4
8
6
4
3
2
10
-5
10
0
8
6
4
3
2
10
-1
8
6
4
3
2
0 cor50_sat_h2vj25_17b_dm_vs_bcru cor50_sat_h2vj25_17b
Cru_2009_003_265r2 lat -3.6
o
2 4 h = 2097 km
[H
2
] = 3.8 X 10
16
cm
-2
6 v
8 10 12 14
Hydrocarbons and H
2
O
Vertical distribution
Mixing ratios on a pressure scale
Summary
• H
2 vertical structure at low latitude in 2009 shows top of atmosphere temperature of 450 K. H
2
X is non LTE with evidence of non LTE in rotation. Proximal S/C tumble density altitude has been provided to project. On basis of -3.6
o latitude observation.
• There is disagreement with CIRS hydrocarbon results partly stemming from their dependence on the Voyager CH
4
UVS profile. Moses theory also uses the Voyager profile. The UVIS result shows a much more vertically confined CH
4 distribution indicating probable seasonal change in vertical dynamics.
• The UVIS upper limit on H
2
O falls below Moore etal 2006, and at the level of the Moses calculachtion which is based on the
Fru chtgruber etal 1997 observation.
H
2
O issues at Saturn
• The Moore etal calculations assume precipitating H
2
O. This results in a constant vertical mixing ratio. Their nominal rate is
~4.5 X 10 27 s -1 . The UVIS result limits to ~10 26 s -1 , within the range of The Fruchtgruber etal observation.
• Enceladus appears to be an unlikely source at a level of ~10 26 s -1 . Moses etal argue for meteoritic dust.
Ionospheric Reaction Chemistry
• Electron Sink:
H
H
2
H
2
H
2
X
X
( v
( v )
4 )
H
3
H
2
H
H
3
3
e e
H
H
2
X
( v )
H
H
H
H
H
• Development of Activated H
2
X (v):
– Photoelectron excitation, energetic electron excitation, solar fluorescence, electron recombination with H
3
+ three-body recombination, proton charge-exchange
, with H
2
X
Summary
• H
3
+ dominant ionospheres predicted for both forcing conditions
• Energy deposition from Stevens et al. (1993) predicted for heterogeneous electron forcing, solar forcing falls short by 3 orders of magnitude
• Observed H
2
EUV band emissions predicted with heterogeneous electron forcing
• Requirement: H * multi-scattering model to predict excitation of H X (v)
2
• Requirement: H
3
+ fine-structure to predict Trafton et al. (1999) emissions