The serotonin receptor gene HTR5A affects plasma triglyceride
advertisement
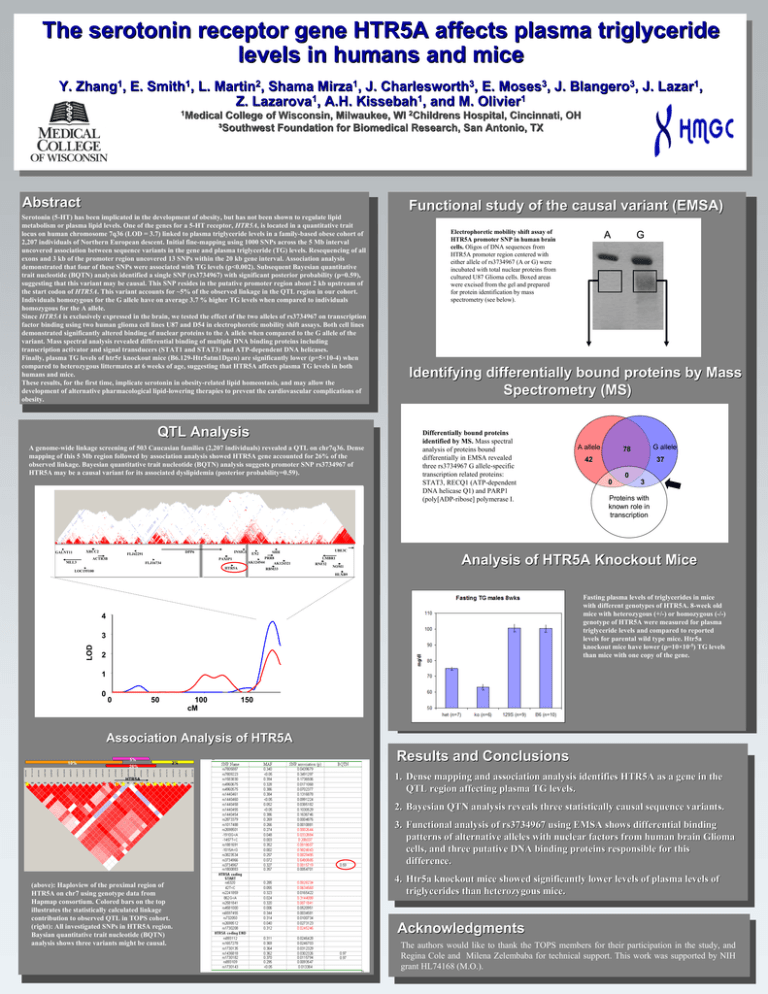
The serotonin receptor gene HTR5A affects plasma triglyceride levels in humans and mice Y. Zhang1, E. Smith1, L. Martin2, Shama Mirza1, J. Charlesworth3, E. Moses3, J. Blangero3, J. Lazar1, Z. Lazarova1, A.H. Kissebah1, and M. Olivier1 1Medical College of Wisconsin, Milwaukee, WI 2Childrens Hospital, Cincinnati, OH ³Southwest Foundation for Biomedical Research, San Antonio, TX Abstract Functional study of the causal variant (EMSA) Serotonin (5-HT) has been implicated in the development of obesity, but has not been shown to regulate lipid metabolism or plasma lipid levels. One of the genes for a 5-HT receptor, HTR5A, is located in a quantitative trait locus on human chromosome 7q36 (LOD = 3.7) linked to plasma triglyceride levels in a family-based obese cohort of 2,207 individuals of Northern European descent. Initial fine-mapping using 1000 SNPs across the 5 Mb interval uncovered association between sequence variants in the gene and plasma triglyceride (TG) levels. Resequencing of all exons and 3 kb of the promoter region uncovered 13 SNPs within the 20 kb gene interval. Association analysis demonstrated that four of these SNPs were associated with TG levels (p<0.002). Subsequent Bayesian quantitative trait nucleotide (BQTN) analysis identified a single SNP (rs3734967) with significant posterior probability (p=0.59), suggesting that this variant may be causal. This SNP resides in the putative promoter region about 2 kb upstream of the start codon of HTR5A. This variant accounts for ~5% of the observed linkage in the QTL region in our cohort. Individuals homozygous for the G allele have on average 3.7 % higher TG levels when compared to individuals homozygous for the A allele. Since HTR5A is exclusively expressed in the brain, we tested the effect of the two alleles of rs3734967 on transcription factor binding using two human glioma cell lines U87 and D54 in electrophoretic mobility shift assays. Both cell lines demonstrated significantly altered binding of nuclear proteins to the A allele when compared to the G allele of the variant. Mass spectral analysis revealed differential binding of multiple DNA binding proteins including transcription activator and signal transducers (STAT1 and STAT3) and ATP-dependent DNA helicases. Finally, plasma TG levels of htr5r knockout mice (B6.129-Htr5atm1Dgen) are significantly lower (p=5×10-4) when compared to heterozygous littermates at 6 weeks of age, suggesting that HTR5A affects plasma TG levels in both humans and mice. These results, for the first time, implicate serotonin in obesity-related lipid homeostasis, and may allow the development of alternative pharmacological lipid-lowering therapies to prevent the cardiovascular complications of obesity. QTL Analysis A genome-wide linkage screening of 503 Caucasian families (2,207 individuals) revealed a QTL on chr7q36. Dense mapping of this 5 Mb region followed by association analysis showed HTR5A gene accounted for 26% of the observed linkage. Bayesian quantitative trait nucleotide (BQTN) analysis suggests promoter SNP rs3734967 of HTR5A may be a causal variant for its associated dyslipidemia (posterior probability=0.59). XRCC2 GALNT11 INSIG1 DPP6 FLJ42291 ACTR3B PAXIP1 MLL3 FLJ16734 HTR5A LOC155100 EN2 A G Identifying differentially bound proteins by Mass Spectrometry (MS) Differentially bound proteins identified by MS. Mass spectral analysis of proteins bound differentially in EMSA revealed three rs3734967 G allele-specific transcription related proteins: STAT3, RECQ1 (ATP-dependent DNA helicase Q1) and PARP1 (poly[ADP-ribose] polymerase I. A allele G allele 78 42 37 0 0 3 Proteins with known role in transcription UBE3C SHH PRR8 AK124544 AK124321 RBM33 Electrophoretic mobility shift assay of HTR5A promoter SNP in human brain cells. Oligos of DNA sequences from HTR5A promoter region centered with either allele of rs3734967 (A or G) were incubated with total nuclear proteins from cultured U87 Glioma cells. Boxed areas were excised from the gel and prepared for protein identification by mass spectrometry (see below). LMBR1 RNF32 NOM1 Analysis of HTR5A Knockout Mice HLXB9 Fasting plasma levels of triglycerides in mice with different genotypes of HTR5A. 8-week old mice with heterozygous (+/-) or homozygous (-/-) genotype of HTR5A were measured for plasma triglyceride levels and compared to reported levels for parental wild type mice. Htr5a knockout mice have lower (p=10×10-5) TG levels than mice with one copy of the gene. 4 LOD 3 2 1 0 0 50 100 cM 150 Association Analysis of HTR5A 10% 5% 26% 2% HTR5A * ** ** *** ** ** Results and Conclusions 1. Dense mapping and association analysis identifies HTR5A as a gene in the QTL region affecting plasma TG levels. 2. Bayesian QTN analysis reveals three statistically causal sequence variants. 3. Functional analysis of rs3734967 using EMSA shows differential binding patterns of alternative alleles with nuclear factors from human brain Glioma cells, and three putative DNA binding proteins responsible for this difference. (above): Haploview of the proximal region of HTR5A on chr7 using genotype data from Hapmap consortium. Colored bars on the top illustrates the statistically calculated linkage contribution to observed QTL in TOPS cohort. (right): All investigated SNPs in HTR5A region. Baysian quantitative trait nucleotide (BQTN) analysis shows three variants might be causal. 4. Htr5a knockout mice showed significantly lower levels of plasma levels of triglycerides than heterozygous mice. Acknowledgments The authors would like to thank the TOPS members for their participation in the study, and Regina Cole and Milena Zelembaba for technical support. This work was supported by NIH grant HL74168 (M.O.).







