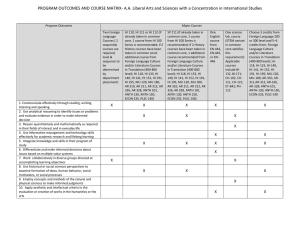
Comparative Mapping of Growth Habit, Plant Height, and Flowering QTLs... Interspecific Families of Leymus
advertisement

Published online November 21, 2006 Comparative Mapping of Growth Habit, Plant Height, and Flowering QTLs in Two Interspecific Families of Leymus Steven R. Larson,* Xiaolei Wu, Thomas A. Jones, Kevin B. Jensen, N. Jerry Chatterton, Blair L. Waldron, Joseph G. Robins, B. Shaun Bushman, and Antonio J. Palazzo Aggressive rhizomes and adaptation to poorly drained alkaline sites, primarily within the western USA, characterize sod-forming L. triticoides (0.3–0.7 m). Conversely, L. cinereus is a tall (up to 2 m) conspicuous bunchgrass adapted to deep well-drained soils from Saskatchewan to British Columbia, south to California, northern Arizona, and New Mexico, and east to South Dakota and Minnesota. Most populations of L. cinereus and L. triticoides are allotetraploids; however, octoploid forms of L. cinereus are typical in the Pacific Northwest. Basin wildrye, and octoploid giant wildrye [L. condensatus (J. Presl) Á. Löve], are the largest cool-season bunch grasses native to western North America. Artificial hybrids of L. cinereus, L. triticoides, and other North American Leymus wildryes display regular meiosis and stainable pollen (Stebbins and Walters, 1949; Dewey, 1972; Hole et al., 1999). Both L. cinereus and L. triticoides are highly self-sterile (Jensen et al., 1990) and hybridize with each other in nature. These species are naturally important forage and hay grasses in the Great Basin and other regions of western North America. Growth habit is a highly variable and ecologically important trait in perennial grasses. Aggressive rhizomes characterize quackgrass [Elymus repens (L.) Desv. ex Nevski] and johnsongrass [Sorghum bicolor (L.) Moench], which rank among the world’s worst perennial grass weeds (Holm et al., 1977). In general, caespitose (i.e., growing in bunches or tufts) and rhizomatous grasses dominate semiarid and mesic grasslands, respectively (Sims et al., 1978). Nutrient islands beneath caespitose grasses may also contribute to clone fitness in this growth form in both mesic and semiarid communities, whereas the distribution of rhizomatous grasses may be restricted to microsites characterized by higher soil organic carbon and nitrogen concentrations (Derner and Briske, 2001). We have observed L. cinereus and L. triticoides growing in close proximity in mixed stands, at several disturbed sites, with no apparent difference in microhabitat. Conversely, we have observed L. triticoides in riparian or wet zones and L. cinereus inhabiting dry adjacent uplands, restricted to seemingly different natural microhabitats. In any case, L. cinereus and L. triticoides display profound differences in growth habit. Lateral branches of L. cinereus grow strictly upward, often within the lower leaf sheaths as tillers, whereas the lateral branches of L. triticoides frequently grow horizontally or downward under the soil surface as rhizomes. In addition to obvious morphological Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. ABSTRACT Leymus cinereus (Scribn. & Merr.) Á. Löve and L. triticoides (Buckley) Pilg. are tall caespitose and short rhizomatous perennial Triticeae grasses, respectively. Circumference of rhizome spreading, proportion of bolting culms, anthesis date, and plant height were evaluated in two mapping families derived from two interspecific hybrids of L. cinereus Acc:636 and L. triticoides Acc:641 accessions, backcrossed to one L. triticoides tester. Two circumference, two bolting, and two height QTLs were homologous between families. Two circumference, seven bolting, all five anthesis date, and five height QTLs were family specific. Thus, substantial QTL variation was apparent within and between natural source populations of these species. Two of the four circumference QTLs were detected in homoeologous regions of linkage groups 3a and 3b in both families, indicating that one gene may control much of the dramatic difference in growth habit between these species. A major height QTL detected in both families may correspond with dwarfing mutations on barley 2H and wheat 2A. The L. cinereus parent contributed negative alleles for all four circumference QTLs, five of nine bolting QTLs, two of five anthesis date QTLs, and one of seven height QTLs. Coupling of synergistic QTL allele effects within parental species was consistent with the divergent growth habit and plant height of L. cinereus and L. triticoides. Conversely, antagonistic QTL alleles evidently caused transgressive segregation in reproductive bolting and flowering time. L wildryes are long-lived Triticeae grasses, closely related to wheat (Triticum spp.) and barley (Hordeum vulgare L.). The genus Leymus includes about 30 species distributed throughout temperate regions of Europe, Asia, and the Americas (Dewey, 1984). More than half of all Leymus species are allotetraploids (2n 5 4x 5 28), but octoploid (2n 5 8x 5 56) and dodecaploid (2n 5 12x 5 84) variants may arise from interspecific hybrids (Anamthawat-Jónsson and Bödvarsdóttir, 2001) or autoduplication within species. These coolseason grasses display remarkable variation in growth habit and stature with unusual adaptation to harsh polar, desert, saline, and erosion-prone environments. Leymus triticoides (creeping or beardless wildrye) and L. cinereus (basin wildrye) are closely related but morphologically divergent North American range grasses. EYMUS S.R. Larson, T.A. Jones, K.B. Jensen, N.J. Chatterton, B.L. Waldron, J.G. Robins, and B.S. Bushman, USDA-ARS, Forage and Range Research Lab., Utah State Univ., Logan, UT 84322-6300; X. Wu, Univ. of Missouri-Columbia, Columbia, MO 65211; A.J. Palazzo, U.S. Army Corps of Engineers, Engineering Research and Development Center-Cold Regions Research and Engineering Lab., Hanover, NH 03755-1290. Received 13 Dec. 2005. *Corresponding author (stlarson@ cc.usu.edu). Abbreviations: AFLP, amplified fragment length polymorphism; ANTH, anthesis date; BOLT, reproductive bolting; CIRC, plant circumference; HGHT, plant height; IM, interval mapping; MQM, multipleQTL model; QTL, quantitative trail locus (i); RFLP, restriction fragment length polymorphism; rMQM, restricted MQM mapping; STS, sequencetagged site; SSR, simple-sequence repeat. Published in Crop Sci. 46:2526–2539 (2006). Genomics, Molecular Genetics & Biotechnology doi:10.2135/cropsci2005.12.0472 ª Crop Science Society of America 677 S. Segoe Rd., Madison, WI 53711 USA 2526 2527 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. LARSON ET AL.: LEYMUS WILDRYES differences in growth habit and stature, L. cinereus and L. triticoides display differences in salt tolerance, seed dormancy, seed color, seed shattering, mineral content, tillering, texture, and other characteristics. The F1 hybrids of L. cinereus and L. triticoides are robust and vigorous plants with relatively large biomass potential compared to other range grasses of western North America. Wu et al. (2003) recently constructed high-density molecular genetic linkage maps for two full-sib families, TTC1 and TTC2. The 164-sib TTC1 and 170-sib TTC2 families were derived from two different L. triticoides 3 L. cinereus F1 hybrids, TC1 and TC2, crossed to different clones of the same L. triticoides tester genotype (T–tester). The TC1 and TC2 F1 hybrids were derived from naturally heterogeneous L. triticoides and L. cinereus seed accessions from Oregon and Alberta, respectively. Together, the TTC1 and TTC2 families capture a snippet of the divergence among and heterogeneity within the L. triticoides and L. cinereus source populations. These two experimental populations provide unique system for gene discovery research and development of breeding markers in perennial grasses. A close relative of quackgrass, Leymus also provides a useful system to study weedy traits such as rhizomes. Our primary objective here was to identify, characterize, and compare QTLs controlling growth habit, plant height, and flowering traits. Another major objective was to compare the location of these Leymus QTLs with genomic regions controlling related traits in other cereal and grass species. MATERIALS AND METHODS Plant Materials and Genetic Maps The full-sib TTC1 and TTC2 molecular genetic maps and pedigrees were described by Wu et al. (2003). The 164-sib TTC1 map included 1069 AFLP markers and 38 anchor loci in 14 linkage groups spanning 2001 cM. The 170-sib TTC2 map contained 1002 AFLP markers and 36 anchor loci in 14 linkage groups spanning 2066 cM. Some 488 homologous AFLP loci and 24 anchor markers, detected in both families, showed similar map order among 14 homologous linkage groups of the TTC1 and TTC2 families (Fig. 1). The 14 homologous linkage groups of the allotetraploid TTC1 and TTC2 families were tentatively numbered according to the seven homoeolgous groups of the Triticeae grasses on the basis of synteny of two or more anchor markers from each of the seven homoeologous groups of wheat and barley. Moreover, genome-specific STS markers were used to distinguish the Ns and Xm marker sequences for homoeologous groups four and five on the basis of similarity to Psathyrostachys juncea (Fisch.) Nevski (genome designation Ns), which is one of the diploid ancestors of allotetraploid Leymus (Wu et al., 2003). Otherwise, homoeologous chromosomes of allotetraploid Leymus were arbitrarily distinguished by the letters “a” or “b.” For example, LG3a is allegedly homoeologous with LG3b (Fig. 1), an assertion supported by synteny among 10 of the 11 anchor markers mapped to these linkage groups (Wu et al., 2003). Additional SSR and STS anchor markers described in the results below were analyzed by methods described by Wu et al. (2003). Detailed genetic maps containing all 1583 AFLP markers mapped in the TTC1 and/or TTC2 families are shown in Supplementary Data files online. For essential comparisons of homology between the Leymus TTC1 and TTC2 families and comparisons of homoeology with other species, we showed all anchor makers but only those AFLP markers that were detected (homologous) in both TTC1 and TTC2 families (Fig. 1). Field Evaluations Ramets from each of the two mapping families (TTC1 and TTC2) were space planted in a randomized complete block (RCB) design with two replicates (blocks) per family at the Utah Agriculture Experiment Station Richmond Farm (Cache Co., UT). Each block contained one entry of each of the 164 TTC1 siblings or one clone from each of the 170 TTC2 siblings plus the parental clones (i.e., TC1, TC2 and T) and single-plant representatives of the heterogeneous L. cinereus Acc:636 and L. triticoides Acc:641 accessions. Individual ramets were transplanted from soil containers (4-cm diameter) in the spring of 2001 to field plots with 2-meter row spacing and 2meter spacing within rows (2-m centers). Plants were aligned among rows such that each plant had four equidistant (2 m) neighboring plants. Each row contained 34 centers (one plant per center), thus blocks were restricted to six or seven rows. The TTC1 rep1, TTC2 rep1, TTC1 rep2, and TTC2 rep2 blocks were arranged lengthwise, respectively, along the 66-m rows to form one continuous array comprised of 24 3 34 centers (46 3 66 m). Quantitative traits, described below, were measured in 2002, 2003, and 2004. Rhizome proliferation was measured as plant circumference (CIRC) in late spring or early summer. For caespitose plants, this could be simply measured by stretching a tape ruler around the tussock at the soil surface. The circumference of irregular sods, characteristic of the more rhizomatous plants, was approximated as the perimeter of a polygon where the corners represent the outermost rhizome branches (i.e., the shortest distance around the outermost rhizomes) at the soil surface. Anthesis date (ANTH) was measured as the number of days from 1 January until the first day of anther extrusion, which is most apparent on warm dry mornings, beginning midJune. In practice, the first day of anther extrusion was interpolated between two or three observations per week. A priori, one unexpected phenomenon in the TTC1 and TTC2 families was variation in reproductive bolting (BOLT), where some genotypes flowered and some did not, first observed in 2002. Individual clones were retrospectively rated as 0 (not flowered) or one (flowered), after seed harvest and mowing, based on ANTH notes take in 2002. We subsequently rated individual plants for the proportion of bolting culms from 0 (no spikes produced) to 1 (virtually all major culms bolting), before seed harvests and mowing, in 2003 and 2004. Plant height (HGHT)as measured after anthesis using a 2-m ruler. Plant height (i.e., actual culm length from soil surface to the spike terminal) was not measured on plants that did not flower. Data Analysis QTL analyses were based on trait means from two replicants for each of the 164 TTC1 and 170 TTC2 segregates in 2002, 2003, and 2004. QTL analyses were also performed on averages over all 3 yr, using output from the LSMEANS procedure of SAS (Statistical Analysis Systems Institute Inc., Cary, NC). Coefficients of skewness and kurtosis, g1, and g2 were calculated as described by Snedecor and Cochran (1980). Departures from normality were deemed significant if they exceeded two standard errors of skewness (SES) and kurtosis (SEK) estimated by (6/N)22 and (24/N)22, respectively (Tabachnick and Fidell, 1996). Histograms of phenotypic values Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 2528 CROP SCIENCE, VOL. 46, NOVEMBER–DECEMBER 2006 (clonal averages) from 2002, 2003, and 2004 were also prepared for CIRC, BOLT, ANTH, and HGHT (Supplementary Data). Broad-sense heritabilities within years were obtained using a SAS program for estimating heritability from lines evaluated in an RCB design in a single environment (Holland et al., 2003). Likewise, heritabilities among years were obtained using another SAS program for estimating heritability from lines evaluated in RCB designs in multiple environments (Holland et al., 2003), modified to account for repeated measurements on perennial plants. All class variables (i.e., rep, clone, year) were treated as random effects. Genotypic and phenotypic correlations between traits evaluated using a SAS program for estimating correlations from RCB designs in multiple environments (Holland et al., 2003), also modified to account for repeated measurements. The original SAS programs for estimating heritabilities, genotypic correlations, and phenotypic correlations (Holland et al., 2003) are available at http://www4.ncsu.edu/|jholland/homepage_files/ Page571.htm (verified 24 July 2006). A sequential and reiterative procedure of QTL detection was performed using the MAPQTL five package (Van Ooijen, 2004). Genome-wide interval mapping (IM) (Lander and Botstein, 1989; Van Ooijen, 1992) was performed in 1-cM increments to identify putative QTLs and possible cofactors Fig. 1. Continued on next page. used in a multiple-QTL model (MQM) (Jansen, 1993; Jansen, 1994; Jansen and Stam, 1994). Markers with the highest loglikelihood ratios (i.e., LOD test statistics) for each QTL (no more than one per chromosome) were selected as the initial set of possible MQM cofactors. A backward elimination procedure was applied to this initial set of cofactors using a conservative significance level of 0.001 to ensure the independence of each cofactor. A reiterative process of restricted MQM (rMQM) mapping, which excludes any syntenous cofactors (i.e., cofactors located on the same linkage group that is being scanned), was used to refine the location of rMQM cofactors (QTLs) and identify new rMQM cofactors (QTLs). Moreover conventional MQM mapping was used to detect (or exclude) syntenous QTLs, where that possibility was apparent. Finally, all putative QTLs were also evaluated using the nonparametric Kruskal–Wallis rank sum test, equivalent to the two-sided Wilcoxon rank sum test for two genotype classes (this study), which means that no assumptions are being made for the probability distributions of the quantitative traits (Lehmann, 1975). A threshold of 3.3 LOD was used throughout these QTL, MQM, and rMQM detection procedures as a close approximation for a genome-wide 5% significance level as determined from simulation tables based on genome size and family type Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. LARSON ET AL.: LEYMUS WILDRYES Fig. 1. Continued on next page. 2529 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 2530 Fig. 1. Continued on next page. CROP SCIENCE, VOL. 46, NOVEMBER–DECEMBER 2006 2531 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. LARSON ET AL.: LEYMUS WILDRYES Fig. 1. Summary and comparison of plant height, growth habit, and flowering QTLs detected in the full-sib Leymus triticoides 3 (L. triticoides 3 L. cinereus) TTC1 and TTC2 families based on 2002, 2003, 2004, and average measurements. The approximate location of QTLeffects (LOD 3.3), corresponding to a genome-wide P # 0.05 signficance level, detected using a restricted multiple QTL model (rMQM) are indicated by black or white bars for each trait 3 year. The updated molecular genetic linkage maps include 488 homologous AFLP markers and mapped in both TTC1 and TTC2 families (Wu et al., 2003), 50 anchor markers (larger bold marker text) mapped in TTC1 and/or TTC2 families (Wu et al., 2003), and 17 additional anchor markers (larger bold italic marker text) mapped in the TTC1 and/or TTC2 families. Annotation next to each anchor marker indicate homoeologous groups of barley (H), wheat (ABD), cereal rye (R), and in parentheses oat chromosome designations. Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 2532 CROP SCIENCE, VOL. 46, NOVEMBER–DECEMBER 2006 (Van Ooijen, 1999) and empirical thresholds based on permutation analyses with 1000 replications (Churchill and Doerge, 1994) specific to each trait. Both methods (Van Ooijen, 1999; Churchill and Doerge, 1994) produced remarkably similar results for all four traits evaluated in this study. Genome-wide IM and rMQM LOD scans based on 2002, 2003, 2004, and average phenotypic values were performed for CIRC, BOLT, ANTH, and HGHT using MapChart version 2.1 (Voorrips, 2002) and the updated Leymus TTC1 and TTC2 genetic maps as described in the results section below. Detailed graphs of each genome-wide LOD scan using the complete high-density linkage maps are provided in the Supplementary Data Online. RESULTS Molecular Map Update A subset of 48 CSU or KSU wheat SSR primer pairs described by Yu et al. (2004), the HVCABG SSR primers described by Becker and Heun (1995), and the HVM068 SSR primers described by Liu et al. (1996) were tested for amplification and polymorphism among the parental genotypes. Seven of these SSR primer pairs (CNL45, KSU154, CNL39, HVCABG, HVN068, KSU149, and KSU171) detected 11 loci in the Leymus TTC1 and/or TTC2 families (Fig. 1). Likewise, another subset of 48 published STS primer pair sequences (Mano et al., 1999; Taylor et al., 2001; Lem and Lallemand, 2003), three sets of STS primers designed from the wheat VRN2 (Yan et al., 2004), and one set of STS primers designed from the BCD1117 barley cDNA clone (Heun et al., 1991) were also tested for amplification and polymorphism among the parental genotypes. Seven of these STS primer pairs (TRX1, TRX2, BCD1117, MWG2230, VRN2, MWG2264, and ADP) detected 13 loci in the Leymus TTC1 and/or TTC2 families (Fig. 1). The thioredoxin h RFLP marker, Xbm2, maps near the self-incompatibility gene locus, S, on homeologous group 1R of Secale (Korzun et al., 2001), and thioredoxin h STS markers have been utilized in perennial ryegrass (Taylor et al., 2001) and Kentucky bluegrass (Patterson et al., 2005). The thioredoxin h STS primers used by Patterson et al. (2005) amplified two distinct sets of sequences, designated TRX1 (AY943821 and AY943822 from L. cinereus Acc:636; AY943823 and AY943824 from L. triticoides Acc:641) and TRX2 (AY943825-AY943827 from L. cinereus Acc:636; AY943828-AY943830 from L. triticoides Acc:641). We designed TRX1 and TRX2 primers that would amplify corresponding sequences from L. cinereus but not L. triticoides—thus providing informative polymorphisms for mapping in the TTC1 and TTC2 backcross families. The TRX2 amplicons mapped to homologous positions of the LG6b linkage group, evident by the coliniearity with homologous TTC1 and TTC2 AFLP markers (Fig. 1). The TRX1 primers amplified 289 bp sequences that mapped to LG1a in TTC1 and LG2b in TTC2 (Fig. 1). The TRX1 locus on LG1a is presumably homeologous to the Xbm2 locus on Secale 1R. The role of thioredoxin h genes in seed germination, self-incompatibility, grain quality, and characteristics has been evaluated in cereals, grasses, and other plants (Juttner et al., 2000; Langridge et al., 1999; Li et al., 1994, 1997; Sahrawy et al., 1996). Of special interest with regard to flowering traits, the VRN1 and VRN2 genes are the two most potent genes controlling differences in vernalization requirement between winter annual and spring annual barley, wheat, and rye (Dubcovsky et al., 1998), and both of these genes have been cloned (Yan et al., 2003, 2004). The VRN2 gene is present on homoeologous regions of 4H in winter barleys (evidently absent in most spring barleys) and a 4A/5A translocation region of Triticum monococcum 5A (Dubcovsky et al., 1998; Yan et al., 2004). Primers designed from the wheat VRN2 gene amplified mixed sequences from Leymus that were very similar in size (supplemental data) and sequence (DQ486013) to the wheat VRN2 gene. These putative Leymus VRN2 sequences map to a locus on the distal long arm of Leymus 5Ns (Fig. 1), which evidently corresponds to the VRN2 locus on Triticum monococcum 5A. Although we have not yet mapped the Leymus VRN1 sequences, this gene is closely associated with the CDO504 marker in other Triticeae species and also maps to Leymus LG5Ns and LG5Xm (Fig. 1) (Wu et al., 2003). In summary, seven SSR primer pairs and seven STS primer pairs (supplemental data) detected 15 additional anchor loci in the TTC1 family and nine additional anchor loci in the TTC2 family, not previously described by Wu et al. (2003). The updated TTC1 map includes 1069 AFLP markers and 53 anchor loci in 14 linkage groups spanning 2001 cM. The updated TTC2 map contained 1002 AFLP markers and 45 anchor loci in 14 linkage groups spanning 2066 cM. Some 488 homologous AFLP loci and 31 anchor markers have been mapped in both families, showing similar map order. Thus, 1583 AFLP markers and 67 different anchor loci have been mapped into 14 linkage groups, which evidently correspond to the 14 chromosome pairs of allotetraploid Leymus (2n 5 4x 5 28). The map locations of the 17 new anchor loci (24 total including seven homologous pairs) (Fig. 1) were largely consistent with the other anchor loci previously mapped in Leymus (Wu et al., 2003). Growth Habit The L. cinereus Acc:636 and L. triticoides Acc:641 progenitor accessions displayed very different levels of rhizomatous spreading, which became more pronounced each year (Table 1). Although CIRC measurements of the TC1 and TC2 F1 hybrids were significantly greater than L. cinereus Acc:636, these means were wellbelow the midparent, TTC1, and TTC2 averages especially in 2004 (Table 1). Circumference of plant (rhizome) spreading showed relatively strong broad-sense heritabilities in the TTC1 family and moderate heritabilities in the more rhizomatous TTC2 family (Table 2). The CIRC trait also showed especially strong heritabilities over years, in both TTC1 and TTC2 families; however, this is expected because plant spreading is a cumulative trait. Not surprisingly, the ratio of genotype 3 year to phenotypic variance was negligible (0.02) for the CIRC trait. The CIRC trait was weakly correlated with the BOLT and ANTH flowering 2533 LARSON ET AL.: LEYMUS WILDRYES Table 1. Trait means 6 SD for circumference of plant spreading (CIRC), proportion of bolting culms (BOLT), anthesis date (ANTH), and plantheight (HGHT) for Leymus cinereus Acc:636, L. triticoides Acc:641, L. triticoides T parental genotype, interspecific TC1 and TC2 parental hybrids, and full-sib L. triticoides 3 (L. triticoides 3 L. cinereus) TTC1 and TTC2 mapping families. Trait Year Acc:636 (n 5 18)† Acc:641 (n 5 15)† t tester (n 5 13)‡ TC1 (n 5 14)‡ TC2 (n 5 14)‡ TTC1 (n 5 164)§ CIRC 2002 2003 2004 Avg. 2002 2003 2004 Avg. 2002 2003 2004 Avg. 2002 2003 2004 Avg. 23 6 10 51 6 26 76 6 21 57 6 23 0.28 6 0.46 0.63 6 0.43 0.85 6 0.33 0.60 6 0.58 176.8 6 2.1 171.1 6 5.1 178.3 6 3.9 175.9 6 2.4 99.0 6 39.8 135.6 6 23.0 182.9 6 18.5 142.2 6 26.0 354 6 186 594 6 326 816 6 416 702 6 208 0.67 6 0.48 0.56 6 0.32 0.78 6 0.35 0.74 6 0.24 173.3 6 2.6 169.3 6 1.4 172.6 6 1.6 172.2 6 1.1 94.5 6 12.6 98.5 6 8.5 123.3 6 12.7 107.3 6 8.7 487 6 61 853 6 120 1263 6 177 653 6 112 1.00 6 0.00 0.98 6 0.06 1.00 6 0.00 0.99 6 0.03 172.0 6 0.0 169.0 6 0.0 173.0 6 0.0 171.3 6 0.0 100.8 6 5.7 100.9 6 5.9 125.7 6 3.4 109.2 6 2.4 96 6 35 200 6 32 256 6 39 183 6 28 0.79 6 0.43 0.88 6 0.19 0.84 6 0.19 0.83 6 0.14 173.6 6 2.1 169.0 6 0.0 175.9 6 4.0 172.8 6 1.3 145.8 6 15.9 155.1 6 12.6 184.7 6 12.3 161.8 6 11.2 125 6 37 231 6 38 282 6 53 212 6 40 1.00 6 0.00 0.77 6 0.21 0.81 6 0.23 0.85 6 0.09 173.1 6 1.5 169.0 6 0.0 175.9 6 4.0 172.6 6 1.5 141.8 6 10.6 138.4 6 13.3 164.6 6 8.7 148.3 6 8.3 180 6 91 288 6 122 362 6 137 273 6 112 0.57 6 0.43 0.33 6 0.24 0.55 6 0.26 0.49 6 0.26 173.5 6 1.9 172.1 6 5.7 175.7 6 4.6 174.5 6 3.5 103.9 6 13.8 108.3 6 12.9 134.8 6 13.8 116.1 6 12.5 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. BOLT ANTH HGHT TTC2 (n 5 168)§ 299 449 548 430 0.73 0.36 0.67 0.59 173.8 171.3 174.1 173.4 109.7 106.1 137.8 117.9 6 116 6 154 6 170 6 144 6 0.38 6 0.25 6 0.27 6 0.26 6 4.8 6 4.9 6 3.7 6 4.0 6 13.0 6 12.4 6 11.8 6 10.7 Measurements expressed in cm (CIRC and HGHT), proportion (BOLT), and days from January one (ANTH). † Sample size based on individual plants. ‡ Sample size based on individual clones of each parental genotype. § Sample size based on means of two clones for each genotype (n). traits, in the TTC1 and/or TTC2 families, but virtually independent from plant height in both TTC1 and TTC2 families (Table 3). At least three significant CIRC QTLs were detected in each family; however, not more than two QTLs were significant in any one year and population (Table 4, Fig. 1). The strongest, most consistent CIRC QTLs in both TTC1 and TTC2 families were located in homologous regions of LG3a (Table 4, Fig. 1). Likewise, homologous CIRC QTLs were also detected on LG3b in both TTC1 and TTC2 families (Fig. 1). The LG3a and LG3b CIRC QTLs, detected in both TTC1 and TTC2 families, are all located near homoeologous copies of VP1 gene markers (Fig. 1). The transcription factor Viviparous-1 (encoded by the Vp1 gene) induces and maintains seed dormancy (McCarty et al., 1991) and has been mapped to orthologous loci in wheat, maize, and rice (Bailey et al., 1999).Inadditiontothehomologousandpossiblyhomoeologous QTLs detected on LG3a and LG3b, in both TTC1 and TTC2 families, a TTC1-specific CIRC QTL was detected on LG6a and a TTC2-specific CIRC QTL was detected on LG5Xm (Fig. 1). Thus, two CIRC QTLs were common to both TTC1 and TTC2 families and two were unique to only one family. The L. cinereus alleles had negative effects at all four (LG3a, LG3b, LG5Xm, and LG6a) CIRC QTLs (Table 4, Fig. 1) consistent with divergent phenotypes of the parental species. Although significant skewness was detected in several evaluations, all four CIRC QTLs (Table 4, Fig. 1) were highly significant (P , 0.0001) on the basis of the nonparametric Kruskal–Wallis rank sum test. Flowering Traits The BOLT and ANTH traits showed relatively strong negative genotypic correlations in both TTC1 and TTC2 families (Table 3), evidently controlled by several common (probably pleiotropic) QTLs (Fig. 1). Thus, data from these two traits are presented together in one section. The L. cinereus Acc:636 and L. triticoides Acc:641 progenitor accessions; F1 interspecific hybrids (TC1 and TC2); and TTC1 and TTC2 families displayed similar BOLT and ANTH phenotypic means (Table 1), except for the fact that L. cinereus showed relatively weak bolting in the first establishment year. Leymus cinereus has a relatively large stature, requiring several years to reach reproductive maturity. Yet, the TTC1 and TTC2 families displayed transgressive BOLT and ANTH segregation that persisted through 2002, 2003, and 2004 (see trait distributions in Supplemental Data). Unlike the F1 hybrids or parental source populations, some transgressive progeny failed to flower in 2002, 2003, 2004, and 2005. The BOLT and ANTH traits both showed relatively weak plot-mean heritabilities over years (Table 2). The ratio of genotype 3 year interaction to phenotypic variance was substantially greater for ANTH (0.18) and BOLT (0.21) compared with CIRC (0.02) or HGHT (0.13), which explains the low plot-mean heritabilities Table 2. Estimates of broad-sense heritability (H 2) 6 SE based on a plot basis and, in parentheses, genotypic mean basis for circumference of plant spreading (CIRC), proportion of bolting culms (BOLT), anthesis date (ANTH), and plant height (HGHT) in the full-sib Leymus triticoides 3 (L. triticoides 3 L. cinereus) TTC1 and TTC2 mapping families. Trait Family 2002 2003 2004 Over years CIRC TTC1 TTC2 TTC1 TTC2 TTC1 TTC2 TTC1 TTC2 0.72 6 0.04 (0.84 6 0.03) 0.54 6 0.06 (0.70 6 0.05) 0.44 6 0.06 (0.61 6 0.06) 0.43 6 0.06 (0.61 6 0.06) 0.50 6 0.07 (0.67 6 0.07) 0.73 6 0.04 (0.84 6 0.03) 0.69 6 0.05 (0.81 6 0.03) 0.66 6 0.05 (0.79 6 0.03) 0.77 6 0.03 (0.87 6 0.02) 0.55 6 0.06 (0.71 6 0.05) 0.63 6 0.05 (0.77 6 0.04) 0.64 6 0.05 (0.78 6 0.04) 0.42 6 0.08 (0.59 6 0.08) 0.43 6 0.08 (0.60 6 0.07) 0.51 6 0.07 (0.68 6 0.06) 0.53 6 0.06 (0.69 6 0.06) 0.73 6 0.04 (0.85 6 0.02) 0.57 6 0.05 (0.73 6 0.04) 0.64 6 0.05 (0.77 6 0.04) 0.69 6 0.04 (0.81 6 0.03) 0.51 6 0.06 (0.67 6 0.06) 0.46 6 0.07 (0.63 6 0.06) 0.73 6 0.04 (0.84 6 0.03) 0.59 6 0.05 (0.75 6 0.04) 0.64 6 0.04 (0.91 6 0.01) 0.50 6 0.05 (0.86 6 0.03) 0.38 6 0.04 (0.76 6 0.04) 0.41 6 0.04 (0.78 6 0.03) 0.31 6 0.05 (0.71 6 0.05) 0.41 6 0.04 (0.78 6 0.03) 0.54 6 0.04 (0.86 6 0.02) 0.48 6 0.04 (0.82 6 0.03) BOLT ANTH HGHT 2534 CROP SCIENCE, VOL. 46, NOVEMBER–DECEMBER 2006 Table 3. Genotypic and in parentheses phenotypic trait correlations 6 SE for circumference of plant spreading (CIRC), proportion of bolting culms (BOLT), anthesis date (ANTH), and plant height (HGHT) in the full-sib Leymus triticoides (L. triticoides 3 L. cinereus) TTC1 (below diagonal) and TTC2 (above diagonal) families. Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. CIRC BOLT ANTH HGHT CIRC BOLT ANTH HGHT — 0.22 6 0.08 (0.15 6 0.05) 20.33 6 0.09 (20.22 6 0.05) 0.01 6 0.09 (0.04 6 0.06) 0.24 6 0.09 (0.13 6 0.05) — 20.81 6 0.06 (20.48 6 0.03) 0.32 6 0.09 (0.40 6 0.04) 20.22 6 0.09 (20.10 6 0.05) 20.83 6 0.04 (20.56 6 0.03) — 0.07 6 0.11 (20.19 6 0.05) 0.12 6 0.09 (0.02 6 0.05) 0.32 6 0.09 (0.36 6 0.04) 20.26 6 0.09 (20.32 6 0.04) — over years for the ANTH and BOLT flowering traits. Although, BOLT and ANTH traits show significant genotype 3 year interaction and the significance of QTLs vary by year (Tables 4), several QTLs were detectable over years (Fig. 1). One notable exception was a highly significant TTC2 ANTH QTL on LG6a in 2002, which was virtually nonexistent in subsequent years. Bolting deficiencies were strongly correlated with late flowering and weakly correlated with short caespitose characteristics (Table 3). A total of nine BOLT QTLs were detected in the TTC1 and/or TTC2 families (Table 4, Fig. 1). The TTC1 and TTC2 BOLT QTL peaks on the upper portion of LG4Xm overlap well enough (Fig. 1) that we counted these as one homologous QTL. Likewise, we believe that there is insufficient separation of the TTC1 and TTC2 BOLT QTLs on LG6a to declare these as different (Fig. 1). Thus, L. cinereus contributed five negative TTC1 and/or TTC2 BOLT QTL alleles and four positive TTC1 and/or TTC2 BOLT QTL alleles (Table 4, Fig. 1). Two of these nine BOLT QTLs were significant in both TTC1 and TTC2 families (i.e., homologous), whereas seven QTLs were unique to TTC1 or TTC2. Four QTLs explained up to 43.9% of the BOLT variation in the 2003 TTC2 data set, which was the most explanatory QTL model in this study (Table 4). A total of five significant ANTH QTLs were detected in the TTC1 or TTC2 families (Table 4, Fig. 1). All five significant ANTH QTLs were unique to TTC1 or TTC2 (Table 4 and Fig. 1). However, the TTC1 family displayed Table 4. Summary of QTL effects detected using multiple QTL model (MQM) scans of circumference of plant spreading (CIRC), proportion of bolting culms (BOLT), anthesis date (ANTH), and plant height (HGHT) in the full-sib Leymus triticoides 3 (L. cinereus 3 L. triticoides) TTC1 and TTC2 backcross mapping families. Trait Family Position (interval†) CIRC TTC1 3a (125–137) 3b (111–112) 6a (107–144) Overall R2 3a (111–161) 3b (111–144) 5Xm (25–66) Overall R2 3a (27–80) 4Xm (11–42) 6a (61–88) 6b (95–117) 7b (11–15) Overall R2 1b (47–85) 4Ns (0–24) 4Ns (95–142) 4Xm (23–41) 4Xm (68–109) 6a (80–125) Overall R2 2a (121–139) 6b (108–127) Overall R2 4Ns (87–124) 4Xm (32–34) 6a (108–127) Overall R2 2a (30–105) 2b (130–164) 5Ns (42–44) 5Xm (0–11) 6b (0–24) 2 Overall R 2a (37–103) 3a (20–34) 3a (77–89) 5Xm (6–82) Overall R2 TTC2 BOLT TTC1 TTC2 ANTH TTC1 TTC2 HGHT TTC1 TTC2 2 2 2 2002 LOD (R , effect‡) 2003 LOD (R , effect‡) 2004 LOD (R , effect‡) NS NS NS NS 4.36 (10.2%, 275)§ 3.56 (8.4%, 268)§ NS 19.7% NS 3.17 (7.4%, 20.24)§ 3.57 (8.4%, 10.25)§ NS 3.20 (7.0%, 10.24)§ 25.7% NS 3.78 (8.7%, 20.25)§ 4.36 (10.8%, 10.26)§ NS NS NS 19.9% NS NS NS 3.54 (7.6%, 22.7)§ 3.70 (7.5%, 12.7)§ 5.85 (15.6%, 23.9)§ 30.9% 7.66 (23.5%, 113.3)§ NS NS NS NS 20.6% 8.56 (17.5%, 111.1)§ 4.10 (8.7%, 17.74)¶ NS 5.64 (11.1%, 28.8)§ 34.1% 3.88 (10.7%, 279)§ 3.47 (8.5%, 273)¶ NS 16.9% 5.15 (12.0%, 2107)§ NS 3.84 (8.7%, 294)§ 21.0% NS 4.12 (10.9%, 20.16)§ NS NS NS 10.9% 5.38 (8.9%, 20.15)§ NS 4.53 (9.0%, 10.16)§ 4.05 (7.2%, 20.13)§ 4.61 (8.1%, 20.14)¶ 7.33 (15.4%, 10.20)§ 43.9% NS NS NS 5.21 (15.9%, 24.5)§ NS NS 13.7% 6.74 (15.2%, 110.1)§ NS 3.44 (7.4%, 17.0)¶ NS 3.86 (11.7% 19.1)§ 31.4% 4.20 (9.3%, 17.6)§ 4.15 (9.2%, 17.5)¶ 3.42 (7.2%, 16.7)§ NS 28.8% 3.16 (9.3%, 283)§ NS 4.08 (9.9%, 287)§ 18.4% 3.9 (9.0%, 2102)§ NS 4.76 (12.1%, 2121)§ 20.3% 6.12 (13.3%, 10.19)§ 5.12 (11.4%, 20.18)§ NS 3.47 (9.3%, 20.16)¶ NS 28.9% NS NS 5.65 (15.3%, 10.21)§ NS NS NS 15.3% NS NS NS NS NS NS NS 10.8 (24.5%, 113.6)§ 5.16 (10.7%, 19.0)§ NS NS NS 33.8% 3.50 (8.8%, 17.0)§ NS 4.96 (13.2%, 18.6)§ NS 20.3% 2 Average LOD (R , effect‡) 3.41 (10.0%, 269)¶ NS 3.33 (8.2%, 64.4)§ 16.4% 5.44 (12.8%, 2104)§ NS 4.49 (10.4%, 296)§ 20.4% 4.00 (7.6%, 10.14)¶ 6.33 (13.6%, 20.19)§ 4.01 (8.1%, 10.15)§ 4.11 (10.0%, 20.16)§ NS 35.9% NS NS 5.64 (11.3%, 10.18)§ 4.37 (9.2%, 20.16)§ NS 4.89 (12.4%, 10.18)§ 32.6% 4.59 (11.4%, 12.5)§ 4.00 (9.9%, 12.2)¶ 19.2% 5.42 (14.7%, 23.12)§ NS NS 14.7% 10.36 (21.2%, 111.6)§ 5.07 (9.6%, 17.8)§ NS e 3.77 (7.0%, 26.8) NS 38.8% 6.40 (14.2%, 18.1)§ 3.98 (7.9%, 16.0)§ NS 4.65 (9.1%, 26.9)§ 34.5% † Approximate positions (cM) where LOD scan exceeded 3.3 threshold as shown in Fig. 1. ‡ Mean of heterozygous genotype (L. cinereus and L. triticoides alleles)– mean of homozygous genotypes (two L. triticoides alleles) expressed in centimeters (CIRC and HGHT), proportions (BOLT), or days (ANTH). § Cofactors selected by interval mapping (LOD . 3.3) and backward elimination (significant at the 0.001 level). ¶ Additional cofactors identified by restricted multiple QTL model mapping (LOD . 3.3) and backward elimination. Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. LARSON ET AL.: LEYMUS WILDRYES ANTH LOD values that were nearly significant on a chromosome region homologous to the TTC2 ANTH QTL on LG4Xm (see ANTH LOD scan in Supplemental Data). Similarly, both TTC1 and TTC2 displayed nearly significant ANTH LOD values on homologous regions of LG3b. In any case, L. cinereus contributed three positive and two negative ANTH QTL alleles at the five significant QTLs (Table 4, Fig. 1). The genome-wide LOD scans were similar but not identical for the BOLT and ANTH flowering traits (Fig. 1 and Supplemental Data). The TTC1 BOLT QTL on LG3a had no apparent effects on ANTH. The TTC2 BOLT QTLs on LG4Ns were associated with significant or nearly significant ANTH QTLs. Likewise, the BOLT QTLs on LG4Xm were associated with significant or nearly significant ANTH QTLs in the TTC1 and TTC2 families. The TTC1 ANTH QTL on LG 5Ns was associated with elevated BOLT LOD, albeit less than the 3.3 LOD threshold (Supplemental Data). Interestingly, the TTC1 and TTC2 BOLT QTLs on LG6a were associated with significant ANTH QTLs only in the TTC2 family. The TTC1 BOLT QTL on LG6a was not associated with elevated ANTH LOD values. The latter observation raises some doubt about the putative homology of the TTC1 and TTC2 BOLT QTLs on LG6a (Fig. 1). Finally the TTC1 BOLT QTLs on LG6b and LG7b were closely associated with a significant or nearly significant ANTH QTLs, respectively. Significant skewness and kurtosis was detected for BOLT and ANTH; however, all QTLs (Table 4, Fig. 1) were highly significant (P , 0.0001) on the basis of the nonparametric Kruskal–Wallis rank sum test. Plant Height The L. cinereus Acc:636 plants were substantially taller than the L. triticoides Acc:641 plants in 2003 and 2004 (Table 1). However, the L. cinereus plants require several years to reach full-size; thus, interspecific differences in plant height were not apparent in 2002. Interestingly, the TC1 and TC2 F1 hybrid genotypes displayed greater plant height than the L. cinereus Acc: 636 or L. triticoides Acc:641 reference individuals, especially in 2002 and 2003 (Table 1). The 2004 HGHT means were substantially greater in 2004, which may be attributable to better rainfall (i.e., 2002 and 2003 were severe drought years) and/or longer plant establishment. Heritabilities over years (Table 2) for HGHT were intermediate between CIRC and the two flowering traits (ANTH and BOLT). Likewise, the ratio of genotype 3 year interaction to phenotypic variance for HGHT (0.13) was intermediate between CIRC (0.02) and the flowering traits, ANTH (0.18) and BOLT (0.21). Weak positive genotypic correlations between HGHT and BOLT were detected in TTC1 and TTC2 families, but no other trait correlations with HGHT were detected in both families. We detected a total of seven HGHT QTLs in the TTC1 and/or TTC2 families (Table 4, Fig. 1). The TTC2 HGHT QTL on LG3a was split into two QTLs using a combination of rMQM and unrestricted MQM mapping 2535 (Table 4, Fig. 1). The single largest HGHT QTL was located in homologous regions of LG2a in both TTC1 and TTC2 families. Likewise, overlapping TTC1 and TTC2 HGHT QTLs on LG5Xm were too close to separate (Fig. 1), which we count as one homologous QTL. Moreover, LG5Xm was the only chromosome that contributed negative HGHT QTL alleles from the taller L. cinereus species, which also seems to support our interpretation that these are homologous QTLs in the TTC1 and TTC2 families. Thus, L. cinereus contributed six positive and one negative HGHT QTL alleles in the TTC1 and/or TTC2 families, which is consistent with divergent phenotypes of the parental species. DISCUSSION Leymus Molecular Genetic Maps Seventeen additional anchor markers described in this study support previous linkage group identifications, tentatively numbered according to the seven homoeologous groups of the wheat, barley, and rye Triticeae cereals (Wu et al., 2003). Among the well-characterized Triticeae genomes, researchers have detected one rearrangement in Aegilops longissima Schweinf. & Muschl. (SI genome), 11 in Ae. umbellulata Zhuk. (U genome), 0 in Ae. speltoides Tausch (S genome), seven in Secale cereale L. cultivated ryes (R genome), and two paracentric inversions in H. vulgare cultivated barleys (H genome) relative to Ae. tauschii Coss., the donor of the hexaploid wheat D genome (Devos and Gale, 2000). The A, B, and D genomes of allohexaploid wheat are evidently colinear except for several large reciprocal translocations involving chromosome arms 2BS and 6BS and chromosomes 4A, 5A, and 7B (Devos and Gale, 2000). One of these rearrangements involves a reciprocal translocation between 4AL and 5AL, which includes the VRN2 gene in T. monococcum L. (Dubcovsky et al., 1998; Yan et al., 2004). Interestingly, the location of the VRN2 gene on Leymus LG5Ns evidently corresponds with the location of VRN2 in T. monococcum (Devos et al., 1995; Dubcovsky et al., 1996). Thus, the Leymus Ns and T. monococcum genomes evidently share the same 4AL/5AL translocation arrangement. Although we detected seven instances of marker synteny in Leymus that were not syntenous in other Triticeae species, we have no firm evidence of rearrangements other than the putative 4Ns/ 5Ns translocation that has already been documented in wheat. Incongruent RFLP marker locations can often be attributed to paralogous duplications or, in the case of PCR, priming annealing at nonhomologous loci. Although there is substantial evidence of colinearity between the Leymus Ns, Leymus Xm, wheat A, wheat B, wheat D, and barley H genomes, we cannot exclude the possibility of a few yet undetected rearrangements in Leymus. These are the first QTL analyses conducted using the high-density linkage maps published by Wu et al. (2003). Throughout much of the linkage map, relatively small irregularities in the LOD scans (Supplemental Data) resulted from imperfect genotyping, missing data, and ambiguous marker orders. Nevertheless, these QTL maps 2536 CROP SCIENCE, VOL. 46, NOVEMBER–DECEMBER 2006 provide a useful starting point for high-resolution QTL mapping experiments using six advanced backcross populations that are currently being genotyped and evaluated in clonally replicated field trials. Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. Comparison of TTC1 and TTC2 QTLs Two circumference, two bolting, and two height QTLs were evidently homologous in both TTC1 and TTC2 families. Conversely, two circumference, seven bolting, all five anthesis date, and five height QTLs were unique to TTC1 or TTC2 families. Differences between the TTC1 and TTC2 families can be attributed to differences between the TC1 and TC2 hybrids, which in turn can only be attributed to genetic variation within the L. cinereus Acc:636 and L. triticoides Acc:641 natural germplasm sources. Thus, functionally important QTL variation was apparent within and between natural source populations of these experimental families and species. Growth Habit The coincidence of QTL effects near the VP1 loci on LG3a and LG3b, in both TTC1 and TTC2, suggest that these QTLs may be controlled by homoeologous copies of the one gene. If so, this gene evidently controls much of the dramatic difference in growth habit between L. cinereus, L. triticoides, and perhaps other grasses. The only other CIRC QTLs, on LG5Xm and LG6a, were unique to the TTC1 and TTC2 families, respectively. A preponderance of mapped barley mutations (four of six loci) that affect vegetative axillary development have been localized to chromosome 3HL (minus arm), including absent lower laterals (als), low number of tillers1 (int1), and semi brachytic (usu), which produce fewer tillers, in addition to granum-a (gra-a), which produces significantly more tillers (Babb and Muehlbauer, 2003; Franckowiak, 1996). A recessive gravitropic lazy dwarf gene (lzd) was also mapped to the short arm of barley chromosome 3 (Franckowiak, 1996; Takahashi et al., 1975), but this mutation seems to be more proximal to the centromere than the Leymus LG3a and LG3b growth habit QTLs. Chromosome 3 is highly conserved within the Triticeae (Devos and Gale, 2000). Colinear from end to end with rice chromosome 1, Triticeae group three is also the most conserved of all chromosome groups when compared with rice (La Rota and Sorrells, 2004). Rice chromosome 1 contains putative transmembrane auxin efflux carrier and DNAJ-like genes (International Rice Genome Sequencing Project, 2005), near the Vp1 locus, originally identified in Arabidopsis pin-forming (Gälweiler et al., 1998) and arg1 (altered response to gravity) (Sedbrook et al., 1999) mutants, respectively. However, pin1- and arg1-like sequences are also present in other regions the rice genome. Rhizome (Hu et al., 2003) and tiller angle (Li et al., 1999) QTLs also map to rice chromosome 1, but these rice loci were not located near the rice Vp1 gene. The TTC2 CIRC rMQM QTL on LG5Xm mapped near the TCP-like sequence (TCP2) amplified from L. triticoides rhizome cDNA using primers designed from the teosinte branched one gene (Doebley et al., 1997), BCD1130, BCD1707, and PSR128 loci. The PSR128 marker also maps in the centromere region of maize chromosome 4 near the recessive lazy1 (la1) gene (Lawrence et al., 2004). Lazy maize mutants, allelic to la1, actively grow downward (prostrate) under light (Firn et al., 2000). We found BCD1707 and BCD1130 sequences on rice chromosome 11, using a TBLASTX search (Ware et al., 2002) of the rice genome (International Rice Genome Project, 2005), near another lazy (la) gene (Miura et al., 2003). The centromere and short arm regions of Triticeae group 5 are homoeologous to rice chromosomes 9 and 12, respectively (Van Deynze et al., 1995; La Rota and Sorrells 2004). Rice chromosome 9 contains a major QTL (Ta) for tiller angle (Li et al., 1999). The TTC1 CIRC QTL peak on LG6a is located approximately (minus) 40 cM from the MWG2264 locus. Interestingly, the uniculm (cul2) mutation and MWG2264 loci are both located (minus) 8.8 and 3 cM relative to the cMWG679 locus on barley 6H (Babb and Muehlbauer, 2003; Graner et al., 1991; Graner, 2004). Compared with other barley mutants, uniculm2 (cul2) is unique in that it inhibits formation of axillary meristems and does not produce tillers (Babb and Muehlbauer, 2003). We were not able to identify rhizome QTLs in Leymus that were homeologous to rhizome QTLs of Sorghum and Oryza. Paterson et al. (1995) detected about 12 rhizome QTLs including a conspicuous cluster of QTLs affecting rhizome number, rhizome distance, and seedling tillers on Sorghum linkage group C, which corresponds to wheat group 4 (Draye et al., 2001). The latter Sorghum QTL also corresponds with sucker and stalk number QTL in Saccharum (Jordan et al., 2004), in addition to the Rhz2 gene and 11 other QTLs controlling rhizome proliferation differences between O. sativa L. and O. longistaminata A. Chev. & Roehr. (Hu et al., 2003). We did not detect any plant circumference QTLs on Leymus LG 4Ns or LG4Xm, which include the PRC140 rhizome QTL marker (Draye et al., 2001) derived from a Sorghum LG C and sequences orthologous to the maize teosinte branched one gene (Doebley et al., 1997). The Rhz3 gene and rhizome QTL on Oryza chromosome 4 correspond to rhizome QTLs on Sorghum LG D (Hu et al., 2003) and Saccharum (Jordan et al., 2004). This region of Oryza chromosome 4 and Sorghum LG D evidently corresponds to Triticeae group 2 (Paterson et al., 1995; Van Deynze et al., 1995). We did not detect any significant rhizome QTL effects on Triticeae group 2. Bolting and Anthesis Date The genetic control of flowering time is complex, involving three major groups of genes on all seven Triticeae chromosome groups (Snape et al., 2001). Two of these groups interact with the environment, namely those controlling vernalization (Vrn) and photoperiod (Ppd). The third set of genes controls developmental rate independent of vernalization and photoperiod, so-called “earliness per se” (Eps) genes. A relatively large number of Eps genes have been mapped to all seven homoeologous groups of wheat and/or barley (Laurie et al., 1995; Snape 2537 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. LARSON ET AL.: LEYMUS WILDRYES et al., 2001), several of which may correspond to BOLT and/or ANTH QTLs detected in Leymus. The Vrn genes map to Triticeae groups 1, 4, 5, and 7 (Dubcovsky et al., 1998; Snape et al., 2001). Moreover, the two most potent genes, on groups 4 and 5, have been cloned and characterized (Yan et al., 2003; Yan et al., 2004). Surprisingly, no significant bolting or anthesis date QTLs were associated with the Leymus CDO504 markers on LG5Ns and LG5Xm, which are presumably closely linked with genes that are orthologous to VRN1. We have amplified VRN1 sequences from Leymus but have not yet found polymorphisms needed for mapping. Likewise, no significant bolting or anthesis date QTLs were associated with the VRN2 gene on LG5Ns. A second yet undetected VRN2 gene may or may not be present on the Xm genome. The most conspicuous wheat and barley Ppd genes are located on homoeologous regions of the 2A, 2B, 2D, and 2H short (plus) chromosome arms. Other Ppd genes have also been reported on barley 1H (Laurie et al., 1995) and 6H (Strake and Börner, 1998). We did not detect ANTH or BOLT QTLs on short (minus) arm of Leymus group two chromosomes. The TTC1 and TTC2 families displayed substantial genetic variation for anthesis date and bolting, including genotypes that have not flowered in the field, which was not apparent among the parental species accessions. This transgressive segregation can be explained by a mixture of antagonistic (i.e., positive and negative) QTL alleles from each parental species. Leymus cinereus contributed five positive and four negative BOLT QTLs and three positive and two negative ANTH QTLs. Thus, correspondence of bolting and anthesis date QTLs (and relatively strong negative genotypic correlations) suggest that outbreeding depression (i.e., transgressive genotypes that to flower) was associated with flowering (i.e., lateness) genes under balancing selection. Although population means and standard deviations may not demonstrate significant bolting depression, the fact that a substantial number of progeny failed to flower year after year indicates that some outbreeding depression is occurring. We have not observed this phenomenon within the L. cinereus Acc:636 or L. triticoides Acc:641 natural source populations. Plant Height The HGHT QTLs on LG2a displayed remarkably strong effects and similar map locations in both TTC1 and TTC2 families, suggesting that these QTLs are homologous. The LG2a HGHT QTL was the only major positive HGHT effect associated with chromatin of the much taller L. cinereus species and detected in both TTC1 and TTC2 families. We interpret these results to mean that this is one of the key QTLs controlling relatively large differences in plant height between L. cinereus and L. triticoides. Börner et al. (1999) mapped gibberellin-sensitive gal (GA-less) and gibberellininsensitive gai (GA-ins) mutations about 55 cM apart on barley 2H. The Leymus LG2a HGHT QTL spans a 40 cM interval from the XANTHA locus down below the CNL045 locus (Fig. 1). The XANTHA–CNL045 LG2b interval also includes the cWMG763 locus (Fig. 1), which was mapped to the short (plus) arm of barley 2H about 36 cm distal from the MWG2287 marker in the barley IGRI 3 FRANKA population (Graner et al., 1991; Graner, 2004). The MWG2287 locus is closely linked to the barley gai mutation in the centromeric region of barley chromosome 2H (Börner et al., 1999). We speculate that the gai (sdw3) locus, described by Börner et al. (1999) and Gottwald et al. (2004), may correspond with a major plant height QTL associated with the cluster of DNA markers near the CNL045 locus of LG2a. The CNL045 loci are associated with high-density clusters of markers on Leymus LG2a and LG2b linkage groups, probably caused by reduced recombination near the centromere. Yang et al. (1995) also described a partially dominant GA-ins dwarfing gene on the short arm of chromosome 2A of wheat (Rht21), which could be homeologous to the gai mutation mapped by Börner et al. (1999). In any case, the barley, wheat, and Leymus plant height genes on homeologous group two are evidently different from the so-called “green revolution” GA-ins dwarfing genes of wheat, barley, and rice encode gibberellin response modulators that map to Triticeae group four (Peng et al., 1999). ACKNOWLEDGMENTS This work was supported by joint contributions of the USDA Agriculture Research Service, Utah Agriculture Experiment Station, Department of Defense Strategic Environmental Research and Development Program CS1103 project, and US Army BT25-EC-B09 project (Genetic Characterization of Native Plants in Cold Regions). Trade names are included for the benefit of the reader, and imply no endorsement or preferential treatment of the products listed by the USDA. The authors gratefully acknowledge the technical assistance of Mauro Cardona, Linnea Johnson, Neil Rasmussen, and Ron Reed. REFERENCES Anamthawat-Jónsson, K., and S. Bödvarsdóttir. 2001. Genomic and genetic relationships among species of Leymus (Poaceae: Triticeae) inferred from 18S–26S ribosomal genes. Am. J. Bot. 88:553–559. Babb, S., and G.J. Muehlbauer. 2003. Genetic and morphological characterization of the barley uniculm2 (cul2) mutant. Theor. Appl. Genet. 106:846–857. Bailey, P.C., R.S. McKibbin, J.R. Lenton, M.J. Holdsworth, J.E. Flintham, and M.D. Gale. 1999. Genetic map locations for orthologous Vp1 genes in wheat and rice. Theor. Appl. Genet. 98:281–284. Becker, J., and M. Heun. 1995. Barley microsatellites: Allele variation and mapping. Plant Mol. Biol. 27:835–845. Börner, A., V. Korzun, S. Malyshev, V. Ivandic, and A. Graner. 1999. Molecular mapping of two dwarfing genes differing in their GA response on chromosome 2H of barley. Theor. Appl. Genet. 99: 670–675. Churchill, G.A., and R.W. Doerge. 1994. Empirical threshold values for quantitative trait mapping. Genetics 138:963–971. Devos, K.M., J. Dubcovsky, J. Dvořák, C.N. Chinoy, and M.D. Gale. 1995. Structural evolution of wheat chromosome 4A, 5A, and 7B and its impact on recombination. Theor. Appl. Genet. 91:282–288. Devos, K.M., and M.D. Gale. 2000. Genome relationships: The grass model in current research. Plant Cell 12:637–646. Dubcovsky, J., D. Lijavetzky, L. Appendina, and G. Tranquilli. 1998. Comparative RFLP mapping of Triticum monococcum genes controlling vernalization requirement. Theor. Appl. Genet. 97:968–975. Dubcovsky, J., M.C. Luo, G.Y. Zhong, R. Bransteitter, A. Desai, A. Kilian, A. Kleinhofs, and J. Dvořák. 1996. Genetic map of diploid Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 2538 CROP SCIENCE, VOL. 46, NOVEMBER–DECEMBER 2006 wheat, Triticum monococcum L., and its comparison with maps of Hordeum vulgare L. Genetics 143:983–999. Derner, J.D., and D.D. Briske. 2001. Below-ground carbon and nitrogen accumulation in perennial grasses: A comparison of caespitose and rhizomatous growth forms. Plant Soil 237:117–127. Dewey, D.R. 1972. Cytogenetics of tetraploid Elymus cinereus, E. triticoides, E. multicaulis, E. karatviensis, and their F1 hybrids. Bot. Gaz. 133:51–57. Dewey, D.R. 1984. The genomic system of classification as a guide to intergeneric hybridization with the perennial Triticeae. p. 209–279. In J.P. Gustafson (ed.) Proc. of the 16th Stadler Genetics Symposium. Plenum, New York. Draye, X., Y.R. Lin, X.Y. Qian, J.E. Bowers, G.B. Burrow, P.L. Morrell, D.G. Peterson, G.G. Presting, S.X. Ren, R.W. Wing, and A.H. Paterson. 2001. Toward integration of comparative genetic, physical, diversity, and cytomolecular maps for grasses and grains, using the sorghum genome as a foundation. Plant Physiol. 125: 1325–1341. Doebley, J., A. Stec, and L. Hubbard. 1997. The evolution of apical dominance in maize. Nature 386:485–488. Firn, R.D., C. Wagstaff, and J. Digby. 2000. The use of mutants to probe models of gravitropism. J. Exp. Bot. 51:1323–1340. Franckowiak, J.D. 1996. Revised linkage maps for morphological markers in barley, Hordeum vulgare. Barley Genet. Newsl. 26:9–22. Gälweiler, L., C. Guan, A. Müller, E. Wisman, K. Mendgen, A. Yephremov, and K. Palme. 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282:2226–2230. Graner, A. 2004. GrainGenes map data report: Barley, I3F. In Graingenes: A database for Triticeae and Avena. http://wheat.pw.usda. gov/cgi-bin/graingenes/report.cgi?class=mapdata&name=Barley% 2C%20IxF (verified 24 July 2006). Graner, A., A. Jahoor, J. Schondelmaier, H. Siedler, K. Pillen, G. Fischbeck, G. Wenzel, and R.G. Herrmann. 1991. Construction of an RFLP map of barley. Theor. Appl. Genet. 83:250–256. Gottwald, S., N. Stein, A. Börner, T. Sasaki, and A. Graner. 2004. The gibberellic-acid insensitive dwarfing gene sdw3 of barley is located on chromosome 2HS in a region that show high colinearity with rice chromosome 7L. Mol. Gen. Genom. 271:426–436. Heun, M., A.E. Kennedy, J.A. Anderson, N.L.V. Lapitan, M.E. Sorrells, and S.D. Tanksley. 1991. Construction of a restriction fragment length polymorphism map for barley (Hordeum vulgare). Genome 34:437–447. Hole, D.J., K.B. Jensen, R.R.C. Wang, and S.M. Clawson. 1999. Molecular marker analysis of Leymus flavescens and chromosome pairing in Leymus flavescens hybrids (Poaceae: Triticeae). Int. J. Plant Sci. 160:371–376. Holland, J.B., W.E. Nyquist, and C.T. Cervantes-Marinez. 2003. Estimating and interpreting heritability for plant breeding: An update. Plant Breed. Rev. 22:9–111. Holm, L.G., D.L. Plucknett, J.V. Pancho, and J.P. Herberger. 1977. The world’s worst weeds: distribution and biology. Univ. Press of Hawaii, Honolulu. Hu, F.Y., D.Y. Tao, E. Sacks, B.Y. Fu, P. Xu, J. Li, Y. Yang, K. McNally, G.S. Khush, A.H. Paterson, and Z.K. Li. 2003. Convergent evolution of perenniality in rice and sorghum. Proc. Natl. Acad. Sci. USA 100:4050–4054. International Rice Genome Sequencing Project. 2005. The map-based sequence of the rice genome. Nature 436:793–800. Jansen, R.C. 1993. Interval mapping of multiple quantitative trait loci. Genetics 135:205–211. Jansen, R.C. 1994. Controlling the type I and type II errors in mapping quantitative trait loci. Genetics 138:871–881. Jansen, R.C., and P. Stam. 1994. High resolution of quantitative traits into multiple loci via interval mapping. Genetics 136:1447–1455. Jensen, K.B., Y.F. Zhang, and D.R. Dewey. 1990. Mode of pollination of perennial species of the Triticeae in relation to genomically defined genera. Can. J. Plant Sci. 70:215–225. Jordan, D.R., R.E. Casu, P. Besse, B.C. Carroll, N. Berding, and C.L. McIntyre. 2004. Markers associated with stalk number and suckering in sugarcane colocate with tillering and rhizomatousness QTLs in sorghum. Genome 47:988–993. Juttner, J., D. Olde, P. Langridge, and U. Baumann. 2000. Cloning and expression of a distinct subclass of plant thioredoxins. Eur. J. Biochem. 267:7109–7117. Korzun, V., S. Malyshev, A.V. Voylokov, and A. Börner. 2001. A genetic map of rye (Secale cereale L.) combining RFLP, isozyme, protein, microsatellite and gene loci. Theor. Appl. Genet. 102:709–717. Lander, E.S., and D. Botstein. 1989. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121: 185–199. Langridge, P., U. Baumann, and J. Juttner. 1999. Revisiting and revising the self-incompatibility genetics of Phalaris coerulescens. Plant Cell 10:1826–1827. La Rota, M., and M.E. Sorrells. 2004. Comparative DNA sequence analysis of mapped wheat ESTs reveals the complexity of genome relationships between rice and wheat. Funct. Integr. Genom. 4:34–46. Laurie, D.A., N. Pratchett, J.H. Bezant, and J.W. Snape. 1995. RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter 3 spring barley (Hordeum vulgare) cross. Genome 38:575–585. Lawrence, C.J., Q. Dong, M.L. Polacco, T.E. Seigfried, and V. Brendel. 2004. MaizeGDB, the community database for maize genetics and genomics. Nucleic Acids Res. 32:D393–D397. Lehmann, E.L. 1975 Nonparamentrics. McGraw-Hill, New York. Lem, P., and J. Lallemand. 2003. Grass consensus STS markers: An efficient approach for detecting polymorphism in Lolium. Theor. Appl. Genet. 107:1113–1122. Li, X., N. Paech, J. Nield, D. Haman, and P. Langridge. 1997. Selfincompatibility in the grasses: Evolutionary relationships of the S gene from Phalaris coerulescens to homologous sequences in other grasses. Plant Mol. Biol. 34:223–232. Li, X., J. Nield, D. Hayman, and P. Langridge. 1994. Cloning a putative self-incompatibility gene from the pollen of the grass Phalaris coerulescens. Plant Cell 6:1923–1932. Li, Z., A.H. Paterson, S.R.M. Pinson, and J.W. Stansel. 1999. RFLP facilitated analysis of tiller and leaf angles in rice (Oryza sativa L.). Euphytica 109:79–84. Liu, Z.W., R.M. Biyashev, and M.A. Saghai Maroof. 1996. Development of simple sequence repeat DNA markers and their integration into a barley linkage map. Theor. Appl. Genet. 93:869–876. McCarty, D.R., T. Hattori, C.B. Carson, V. Vasil, M. Lazar, and I.K. Vasil. 1991. The Viviparous–1 developmental gene of maize encodes a novel transcriptional activator. Cell 66:895–905. Mano, Y., B.E. Sayed-Tabatabaei, A. Graner, T. Blake, F. Takaiwa, S. Oka, and T. Kobatsuda. 1999. Map construction of sequence-tagged sites (STSs) in barley (Hordeum vulgare L.). Theor. Appl. Genet. 98:937–946. Miura, K., M. Ashikari, M. Matsuoka, and Y.I.C. Hsing. 2003. Mapping of the lazy gene, la. Rice Genet. Newsl. 20:29–30. Paterson, A.H., K.F. Schertz, Y.R. Lin, S.C. Liu, and Y.L. Chang. 1995. The weediness of wild plants: Molecular analysis of genes influencing dispersal and persistence of johnsongrass, Sorghum halepense (L.). Pers. Proc. Natl. Acad. Sci. USA 92:6127–6131. Patterson, J.T., S.R. Larson, and P.G. Johnson. 2005. Genome relationships in polyploid Poa pratensis and other Poa species inferred from phylogentic analysis of nuclear and chloroplast DNA sequences. Genome 48:76–87. Peng, J., D.E. Richards, N.M. Hartley, G.P. Murphy, K.M. Devos, J.E. Flintham, J. Beales, L.J. Fish, A.J. Worland, F. Pelica, D. Sudhakar, P. Christou, J.W. Snape, M.D. Gale, and N.P. Harberd. 1999. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400:256–261. Sahrawy, M., V. Hecht, J. Lopez-Jarmillo, A. Chueca, Y. Chartier, and Y. Meyer. 1996. Intron position as an evolutionary marker of thioredoxins and thioredoxin domains. J. Mol. Evol. 42:422–431. Sedbrook, J.C., R. Chen, and P.H. Masson. 1999. ARG1 (Altered Response to Gravity) encodes a DNAJ-like protein that potentially interacts with the cytoskeleton. Proc. Natl. Acad. Sci. USA 96: 1140–1145. Sims, P.L., J.S. Singh, and W.K. Lauenroth. 1978. The structure and function of ten western North American grasslands. I. Abiotic and vegetation characteristics. J. Ecol. 66:251–281. Snape, J.W., K. Butterworth, E. Whitechuch, and A.J. Worland. 2001. Waiting for fine times: Genetics of flowering time in wheat. Euphytica 119:185–190. Snedecor, G.W., and W.G. Cochran. 1980. Statistical methods, Seventh ed. Iowa State Univ. Press, Ames, IA. Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. LARSON ET AL.: LEYMUS WILDRYES Stebbins, G.L., and M.S. Walters. 1949. Artificial and natural hybrids in the Gramineae, tribe Hordeae. III. Hybrids involving Elymus condensatus and E. triticoides. Am. J. Bot. 36:291–301. Strake, S., and A. Börner. 1998. Molecular mapping of the photoperiod response gene ea7 in barley. Theor. Appl. Genet. 97:797–800. Tabachnick, B.G., and L.S. Fidell. 1996. Using multivariate statistics (3rd ed.). New York: Harper Collins. Takahashi, R., J. Hayashi, T. Konishi, and I. Moriya. 1975. Linkage analysis of barley mutants. Barley Genet. Newsl. 5:56–60. Taylor, C., K. Madsen, S. Borg, M.G. Møller, B. Boelt, and P.B. Holm. 2001. The development of sequence-tagged sites (STSs) in Lolium perenne L.: The application of primer sets derived from other genera. Theor. Appl. Genet. 103:648–658. Van Deynze, A.E., J.C. Nelson, E.S. Yglesias, S.E. Harrington, D.P. Braga, S.R. McCouch, and M.E. Sorrells. 1995. Comparative mapping in grasses. Wheat relationships. Mol. Gen. Genet. 248:744–754. Van Ooijen, J.W. 1992. Accuracy of mapping quantitative trait loci in autogamous species. Theor. Appl. Genet. 84:803–811. Van Ooijen, J.W. 1999. LOD significance thresholds for QTL analysis in experimental populations of diploid species. Heredity 83:613–624. Van Ooijen, J.W. 2004. MapQTL W 5, Software for the mapping of quantitative trait loci in experimental populations. Kyazma B.V., Wageningen, the Netherlands. 2539 Voorrips, R.E. 2002. MapChart: Software for the graphical presentation of linkage maps and QTLs. Heredity 93:77–78. Ware, D., P. Jaiswal, J. Ni, X. Pan, K. Chang, K. Clark, L. Teytelman, S. Schmidt, W. Zhao, S. Cartinhour, S. McCouch, and L. Stein. 2002. Gramene: A resource for comparative grass genomics. Nucleic Acids. Res. 30:103–105. Wu, X.L., S.R. Larson, Z.M. Hu, A.J. Palazzo, T.A. Jones, R.R.C. Wang, K.B. Jensen, and N.J. Chatterton. 2003. Molecular genetic linkage maps for allotetraploid Leymus (Triticeae). Genome 46:627–646. Yan, L.L., A. Loukoianov, A. Blechl, G. Tranquilli, W. Ramakrishna, P. SanMiguel, J.L. Bennetzen, V. Echenique, and J. Dubcovsky. 2004. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303:1640–1644. Yan, L., A. Loukoianov, G. Tranquilli, M. Helguera, T. Fahima, and J. Dubcovsky. 2003. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 100:6263–6268. Yang, T.Z., X.K. Zhang, H.W. Liu, and Z.H. Wang. 1995. Chromosomal arm location of a dominant dwarfing gene Rht21 in XN0004 of common wheat. p. 839–842. In Z.S. Li and Z.Y. Xin (ed.) Proc. 8th Int. Wheat Genet. Symp. China Agricultural Scientech Press, Beijing. Yu, J.K., T.M. Dake, S. Singh, D. Benscher, W. Li, B. Gill, and M.E. Sorrells. 2004. Development and mapping of EST-derived simple sequence repeat markers for hexaploid wheat. Genome 47:805–818. Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. Genome-wide QTL interval mapping (IM) and restricted multiple QTL model (rMQM) mapping in the full-sib Leymus TTC1 and TTC2 families based on log of the odds (LOD) for circumference of plant spreading (CIRC), proportion of bolting culms (BOLT), anthesis date (ANTH), and plant height (HGHT) based averages over 2002, 2003, and 2004. A threshold value of 3.3 LOD is shown for reference. LG1a--TTC1 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 120 125 130 4 115 P33M61.137 E38M60.108 P35M61.177 E38M49.399 P33M61.233 P33M47.234 P44M62.119 P44M62.368 E37M47.327 E41M49.134 P35M61.174 E38M60.311 E38M60.294 P33M47.273 E36M48.168 E36M49.306 E36M59.250 E38M60.177 E36M48.225 E36M48.069 E41M49.278 E41M59.271 E38M47.222a E41M48.322 P44M62.164a E37M47.270 P42M62.153 E37M61.182 E41M60.361 E36M59.217 E41M47.148 E38M60.162 BCD1150 E41M49.278 E37M62.244 P33M60.290 E36M48.257b E37M62.270 E41M47.119 E38M47.222a 85 110 3 E41M47.148 E38M60.107a P44M49.388 E38M59.101 E38M49.146 90 E41M59.163 E38M60.159 P42M61.114 E37M61.344 95 1H HVA.094 E41M61.122 E37M47.196 P42M61.218 100 P33M62.354 E41M61.398 P35M59.188 P35M62.180 105 E41M59.310 2 E38M49.066 E36M50.397 E36M61.291 P33M60.147 E41M59.335 E41M61.367 P33M60.360 P35M61.361 P33M47.161 E38M60.181 E36M59.167 E36M61.231 E41M61.271 P42M62.293b P33M61.331 E41M48.388 E38M49.396 E37M61.173 E36M49.287 E38M47.293 E37M49.146 E41M59.124 E38M59.215 P33M62.346 E36M61.286 E41M59.193 E36M48.163 E41M47.113 E41M60.123a E41M49.217 E38M49.266 E37M49.361 E37M49.321 E37M60.153 E38M60.074 E38M59.330 E41M59.082 E41M49.072 E37M60.146 E36M50.254 P33M50.281 P33M59.158 E36M61.336 P33M60.290 75 80 P33M50.162 P33M62.220 P42M61.072 E36M61.377 70 E38M49.140 P33M47.280b P33M47.280b P33M50.162 P35M59.213 E38M47.185 E37M61.306 1 E36M61.336 0 E41M49.220 E41M49.072 P35M50.213a 4 65 E37M49.321 3 60 LG1b--TTC2 2 55 1 50 0 45 P33M47.258 4 40 3 35 2 30 1 25 LG1b--TTC1 0 20 4 15 E36M61.254 E38M49.066 E41M59.098 E36M59.167 E36M59.062 E38M60.202 P33M47.258 P33M60.360 P33M47.161 P42M62.187 P42M61.166 P42M62.181 E38M60.182 E37M61.306 E41M49.052 E36M61.292 E41M48.162b P44M62.137 P33M62.346 E36M50.222 E41M47.300 E38M47.253 1R TRX1 E38M49.319 E41M48.110 E36M48.061 P35M60.248a P44M62.266 P35M62.353 P33M50.305 E41M60.094 E37M61.167 E38M49.396 E38M47.293 P44M62.331b E36M49.321 E36M48.271 E37M62.278 E41M48.389 E36M62.240 P44M62.293 E41M60.123a E41M49.217 E41M61.321 3 10 LG1a--TTC2 2 5 1 0 0 1B(A) E38M60.107a E36M59.239 P35M59.211 E41M59.163 P44M62.164b HVA.094 1H E38M59.101 BCD1562 1BD(A) E41M47.110 P33M62.354 P42M61.218 E37M47.196 E38M60.291 E41M61.398 E36M59.296 E41M60.195 P35M61.177 GDM126.146 1D E38M60.108 E41M47.163 P44M62.368 P33M47.234 P44M62.124 P33M62.138 E41M49.134 E37M47.327 E38M60.294 E38M60.311 E41M62.184 P35M50.127 E36M62.363 P35M50.142 E41M47.130 P44M49.228a E41M59.090 E41M47.129 P33M59.251 E41M59.197 E36M61.191 E38M47.394 E41M61.226 P42M62.062 E38M49.283b P35M62.350 E37M60.097 E36M61.240 E36M62.140 E37M60.103 E37M47.319 E37M62.388b P35M61.396 E41M62.173 E38M60.213 E36M62.346 E36M61.323 E41M61.385 E41M48.245 P33M61.198 E37M62.232 E41M48.131 P35M59.154 P44M62.105 P33M61.367 P42M61.117 P33M62.319 E38M47.229 E41M47.355 P33M50.154 E38M59.317 E37M61.095 P33M47.255 E37M62.201b E37M61.290 E37M47.151 E37M47.150 E41M61.252 E37M49.092 E36M61.192 E41M49.171 E36M61.267 E36M61.191 E38M49.283b 1AD WMC336.388 P35M62.350 E41M61.265 E37M47.126 E36M62.140 E37M60.103 P44M62.378 P35M61.394 P44M49.200 P33M61.367 P35M49.334 P35M49.311 P33M61.198 P33M47.328 P35M49.139 P42M61.117 E36M61.323 E41M48.245 E38M60.213 E36M62.346 E41M62.189 E41M61.385 E37M49.051 E37M47.319 E37M47.320 E41M47.355 E41M60.360 E37M49.079 E37M61.073 E36M59.317 E41M61.141 E38M49.269 E37M60.097 E37M61.095 E38M59.204 E41M60.336a E41M47.237 E37M49.092 E36M61.267 P44M62.209 P33M50.246b E38M49.214 P33M62.271 E41M48.053 E37M60.284 P33M59.118a P44M62.351 P33M50.246b E38M49.241 E38M59.153 E38M49.386 E36M62.057 E41M48.147 P44M49.133 P35M61.141 E36M62.192 E41M47.078 E36M62.150 P33M50.180b P44M49.250 E37M60.259 E38M49.241 E38M49.256 P35M60.119 P44M49.133 P42M62.312 P42M61.157 E36M62.192 E41M47.078 E41M62.337 135 E38M49.063a CIRC_IM BOLT_IM ANTH_IM HGHT_IM CIRC_rMQM BOLT_rMQM ANTH_rMQM HGHT_rMQM Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. P44M62.176 E38M60.056 30 E38M60.153 P44M62.228 35 P33M62.117 E41M59.094 P35M62.365 E37M62.072 P42M62.303 P42M62.298 E36M59.221 40 E37M60.172b E41M61.111a E38M59.282 2H XANTHA.395 P44M62.228 45 E36M50.224 E41M61.352 50 60 65 70 75 2A 85 90 P33M48.212 E37M60.192 E41M60.139 2H XANTHA.270 55 80 E36M61.152a E36M50.224 E38M47.180 P33M48.212 P33M50.249 E37M60.192 E41M47.249 E36M59.234 E36M61.361 P42M61.367 E36M49.271 E37M60.342 E41M62.194 E37M47.145 E41M61.188 E37M62.228 E36M61.297 E41M49.092 E36M61.253 P33M61.216 CNL045.154 E37M62.182 E36M48.098 P35M50.385 P33M48.215 E41M48.070 E36M61.361 E36M61.297 E41M61.188 E41M61.254 E41M62.194 E41M48.085a E37M60.342 E37M62.228 E38M60.087 E38M60.069 E41M49.092 P42M61.367 E41M62.319 P33M61.216 P33M60.143 E41M48.062a P33M48.154 P33M61.203 E41M62.152 E41M50.202 E38M59.329 E37M61.232 E37M62.252 E37M62.252 E36M48.248 P33M48.224 95 100 E41M60.123b E41M60.238 P35M50.279 E37M49.102 P33M50.180c E36M48.202 P35M50.279 E36M59.222 E37M49.102 E41M61.099 E41M47.139 E36M49.130 E36M48.356 E36M59.222 105 110 E41M59.318 P33M59.318 P33M62.231 E36M48.356 P33M50.246a E41M59.154 E41M47.356 P42M61.185 E36M49.339 P42M61.122 P42M61.196 135 P33M48.126 P35M50.307 2ABD GWM382.095 P33M62.291 140 E38M49.152 P35M62.111 P35M49.140 P44M62.088a 145 E36M49.348 E36M48.075 E36M49.366 E38M49.067 150 E41M48.112 E36M59.182 E41M47.285 E38M59.074 155 130 160 165 E37M60.188 P35M62.320 E37M61.110 P33M60.130 E37M62.131 E37M60.297 E41M50.159 E37M62.272 E37M60.257 E38M60.280 E41M60.172 E41M49.215 E37M49.304 P35M59.253 P33M48.079 P33M60.249 P35M61.364 P33M61.285 E37M62.183 E36M59.171 E41M48.226 E38M60.234 E37M62.261 E38M60.130 E37M62.136 E38M47.105 E36M49.313 P33M59.241 P42M62.115 E37M61.141 P44M49.223 E36M61.157 E37M49.147 2A CNL045.172 P33M60.180 E41M60.293a E37M47.370 E37M60.294 P33M59.278 P35M60.227 E37M60.257 E36M61.157 E37M62.272 P33M59.241 E37M60.188 P35M60.248b E41M59.257 P33M61.285 P33M60.130 E41M48.251 TRX1 1R E37M62.183 P35M61.364 P33M48.079 P35M59.253 P33M60.250 E37M62.260 E36M59.171 E37M62.212 E36M62.145 E41M49.247 E41M61.350 E37M61.281 E37M61.141 E37M49.147 E36M62.097 P33M60.180 E37M47.143b E36M49.179 P33M59.278 P35M60.280 E41M61.119 P33M59.172 E37M62.269 E41M61.359 E38M47.076a 125 2H MWG763.277 E41M61.099 E38M49.074 120 P42M61.142 E41M61.119 P33M59.172 P33M59.242 115 P33M47.345 P35M60.227 CIRC_IM CIRC_rMQM P35M62.377 E38M47.076a P33M62.231 E41M60.088 E36M62.339 E41M47.357 P33M47.243 P44M49.149 P33M62.296 E41M48.060 P33M62.333 P33M48.126 P35M49.242 E37M49.160 GWM382.095 2ABD P35M62.399 P35M60.144 P33M62.291 E36M49.366 E41M47.132 E41M48.112 E41M47.131 P44M49.369 P42M62.146 E41M47.214 P44M49.323 P33M48.397 P35M50.258 E41M48.188 P33M50.180a P33M50.180a E36M49.266 E41M47.359 E36M61.091 P35M60.272 P42M62.067 P35M60.272 E41M59.323 E37M49.165 E37M49.165 E37M62.269 P35M59.372 P33M60.268 P33M48.350 E37M60.186 P33M48.348 E37M47.198 P33M61.071b E41M61.156 P42M61.081 E38M59.220 E36M49.092 P35M61.243 P33M60.217 P35M49.090 2ABD GWM382.126 P33M62.394 E41M47.155 E38M59.095 E41M60.144 E37M47.356 E38M59.179 P42M61.140 E41M59.126a E38M47.278 P42M61.081 E38M59.220 E36M49.109 E37M47.056 P35M61.243 P44M49.286 P33M60.217 E41M47.155 E38M59.095 E41M60.144 E37M47.356 E38M59.179 P33M50.245 E41M59.126a GWM382.126 2ABD P33M48.067 E38M47.278 P33M48.067 BOLT_IM ANTH_IM HGHT_IM BOLT_rMQM ANTH_rMQM HGHT_rMQM P42M61.140 4 P35M50.208 P33M48.392 3 E36M59.293 E41M47.204 E36M50.311 P44M49.095b P33M50.227 P33M50.152 P35M62.194 P35M62.163 E41M62.160 E37M49.270 P44M49.258 E37M61.102 2 E41M49.131 E36M61.248 1 0 E41M48.241a E36M59.293 E41M49.131 E36M49.311 E36M59.392 E38M47.072 P35M62.194 P33M60.125 P33M50.152 P33M48.328 P35M50.208 P33M48.394 P44M62.299 5 E38M60.153 LG2b--TTC2 4 E36M61.248 3 25 2 20 LG2b--TTC1 1 15 P33M61.175 E38M47.364 E36M48.172 E37M61.246 E41M48.255 E38M60.263 E37M61.155 P33M61.312 P42M61.145 P33M48.139 P35M49.202 P33M61.212 P33M59.256 E38M59.217 P44M62.223 P35M62.178 P44M49.246 E41M59.184 E36M61.221 E38M60.056 P33M62.364 P35M49.297 P35M62.365 E36M48.305 0 10 LG2a--TTC2 6 5 4 3 2 1 0 5 10 9 8 7 6 5 4 3 2 1 0 LG2a--TTC1 P33M62.285 E41M48.255 P33M48.139 P33M61.175 P33M61.312 P33M47.078 P35M62.187 P35M49.202 P33M61.213 P35M61.225 E36M48.172 E38M60.263 E37M61.155 E41M59.077 E38M47.364 E36M61.138 E41M59.345 P44M62.223 E36M61.175 P44M49.246 P33M59.256 E36M50.247 0 LG3a--TTC1 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. E41M48.133 P33M50.328 P33M61.149a E41M61.290 E37M49.132 65 70 P35M59.284 P42M61.316 E37M61.350 E37M60.356a 75 80 P44M49.331 E37M62.274 P35M62.385 E41M61.234a E41M61.235 P33M60.305 E41M60.080b E41M62.354 E41M62.350 E38M49.084 BCD1532 3AB(C) E41M60.111 P33M62.144 3H 110 115 120 125 E37M47.386 P44M49.110 P35M60.074b E38M47.374 E37M49.329 P33M47.167 P33M62.190 E36M62.173 E37M61.199 E37M60.339 E36M61.263 P33M61.153 E36M59.253 P44M49.329 E36M61.097 E37M62.082 E37M60.203 E36M61.141 E37M49.303 E41M61.131 E36M49.351 E36M50.351 E38M60.242 E41M59.113 P35M49.242 E36M61.234 E38M60.316 E37M47.124 E38M47.140 E41M49.163 E38M60.305 E38M47.192 E41M47.225 E38M47.076b P35M62.199 E37M47.302 P33M50.328 P33M61.149a E41M61.290 E36M61.303 E41M61.127 E41M48.133 P35M59.284 130 135 E37M47.224 140 E41M62.069 145 P44M49.139 E37M60.302 150 E37M49.349 P35M50.264 P33M47.097 E37M60.082 E38M60.278 P44M49.324 VP1i5.406 E41M61.220 E37M60.083 P44M62.204 E36M49.248 E37M47.225 E36M59.219 P33M50.146 E36M62.090 E38M47.185 E37M49.141 E36M48.167 P33M61.174 E41M48.248 E37M49.118 P35M49.224 E41M62.341 E37M49.201 E41M61.063 P35M61.089 P35M62.147 E37M60.303 E41M49.232 E37M47.224 E37M49.355 E41M62.069 P35M59.174 170 E38M47.172 E41M60.286 P33M60.207 E41M47.134 P35M62.242 E36M62.227 E41M61.077 P33M50.345 E37M62.162 3ABD 3ABD E41M49.304 P33M60.227 E36M62.227 P33M47.099 VP1i2.394 E37M62.162 E37M47.326 E38M59.249 E36M48.204 E37M47.263 E41M59.206 E41M62.204 P42M62.249 P33M48.155 E36M59.262 E41M47.173 E37M47.326 E36M48.284 E41M62.204 E41M62.260 P42M62.249 P33M59.245 E37M49.179 E41M59.222 E41M59.325b E37M49.358 4B(F) CDO1401 E41M49.285 E41M59.210 E36M50.237a E37M49.358 E38M59.065 E37M62.235 P33M62.090 E37M62.075 P33M60.393 P33M61.129 P44M62.093 160 165 E38M49.064 P33M60.207 E41M49.302 E41M48.102 P33M60.192 P44M49.139 P33M59.118b 155 E36M61.243 P44M62.360 P35M49.270 P33M47.111 P35M61.089 P35M62.147 E38M47.359 E37M61.191 E36M50.170 E36M48.308 P44M62.093 P33M61.129 P33M47.072 P33M47.073 P33M47.167 E37M47.386 P44M49.110 E38M47.374 E37M61.199 E37M60.339 P33M62.190 E37M49.329 P33M61.153 E36M62.173 P33M50.116 E36M49.351 E36M59.253 E36M61.097 E36M61.141 E37M60.203 P44M49.329 E37M60.200b E37M62.082 E36M61.263 E41M61.131 E37M49.303 E36M61.234 E38M47.140 E38M60.242 E37M47.242 E36M48.066 E36M61.206 E38M60.316 P33M47.073 E37M47.225 E36M59.219 E41M59.365 E41M60.296 P33M48.378 P33M50.149 P33M48.119 P33M50.285 P44M49.324 P44M49.197 E41M60.166 P33M47.072 E36M62.203 P44M62.360 105 3H E38M49.057 E36M62.338 E41M60.166 E37M61.349 100 SIP1.413 E41M62.154 SIP1.413 E36M62.338 P33M60.192 E41M61.220 P44M62.204 VP1i5.406 E37M60.083 P35M60.135 P35M50.120 P42M61.197 E38M49.057 E38M47.165 3ABD E41M49.135 E41M47.167 E41M62.223 E36M48.164 P42M61.232 E36M61.311 GWM005.080 3A P35M62.225 E36M62.302 P35M50.154 E41M49.122b E36M48.101 E37M47.226 E38M60.359 E41M47.071 E36M59.287 E36M61.330 90 95 E41M49.135 E41M47.167 P42M61.197 E41M60.343 E41M48.102 P33M47.097 85 P35M50.120 3H SIP1.291 E41M61.095 P35M60.074a P33M59.118b E41M61.107 CIRC_IM ANTH_IM CIRC_rMQM ANTH_rMQM BOLT_IM HGHT_IM BOLT_rMQM HGHT_rMQM 4 60 3 E41M47.071 E41M49.090 E36M61.330 E38M47.076b E41M47.225 2 E38M60.359 55 E41M61.394 P33M62.393 P33M47.179 P33M47.279 P44M62.334 1 E41M62.223 50 E36M61.339a P42M62.177 E36M59.261 P33M62.343 P33M47.310 P33M47.179 P33M47.280a E41M60.156 P33M62.393 CDO460 3ABR(C) E36M49.206 E36M61.092 E41M48.085b E41M61.313a P35M49.082 0 E41M49.122b P33M47.228 4 45 E38M47.228 E41M59.148 LG3b--TTC2 3 3A GWM005.080 P35M49.082 2 3H E41M48.390 1 40 E38M47.214 E36M48.164 P42M61.232 P35M50.314 P35M62.225 SIP1.324 0 35 5 30 4 25 3 20 2 P35M62.385 P44M62.197a P35M61.082 E36M62.130 P44M49.253 E37M62.257 E38M49.084 E37M47.128 E41M59.268 E37M47.305 P33M62.144 E37M62.096 P33M60.308 1 15 LG3b--TTC1 0 10 4 E38M49.363 E38M47.228 P33M47.131 P33M48.194 P35M59.195 P33M60.104 P42M61.294 P42M62.178 E38M59.298a P35M61.158 3 5 LG3a--TTC2 2 P33M50.250 1 0 0 LG4Ns--TTC1 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. P42M62.164 E38M59.346 P35M61.277 E41M60.069 P35M59.112 40 4ABD GWM165.202 50 E38M60.090 E41M61.373 E41M60.069 P33M59.133 E38M59.346 P35M61.277 E41M47.205b E41M60.233 P35M61.370 GWM165.202 4ABD E38M59.387 E41M62.393 E38M47.063 55 60 65 70 75 80 E41M60.132a E36M61.342 E41M49.143 E41M49.142 E41M50.282 E36M49.353 E38M47.103 E36M61.287 E41M61.084b E41M60.196 E41M60.243 E38M60.259 E41M59.342 E41M62.335 E36M62.319 3A CNL039.229 E36M50.376 E41M61.109 P42M61.317 E36M62.249 E36M59.374 E41M48.185a 85 P33M59.302 90 P33M62.070 95 4ABD (F) 4H E41M62.393 E36M49.187 E37M49.316 P33M59.091 E37M49.313 E41M59.152 P33M47.094 E41M49.143 E37M61.186 E41M61.109 E41M48.067 E38M60.259 E37M60.262 E36M61.287 E38M47.103 E36M62.319 E41M62.336 E36M49.376 P33M50.264 E41M61.084b E36M50.376 E41M60.247 E38M60.122 E37M49.174 E36M50.353 E41M60.196 E37M60.325 E36M49.353 P35M62.370 P33M62.070 E36M62.249 E41M59.342 E37M60.183 E37M61.332 E37M62.286 4D P33M62.351 P33M59.134 E41M61.186 E41M49.198b P33M48.399 E41M60.326 P35M50.371 P35M50.372 P35M60.392 E37M47.304 E38M60.325 E41M62.235 E41M62.082 P35M49.187 E41M62.239 P33M48.359 E36M61.378 E41M61.381 E41M47.150 E41M47.301 E41M61.381 E36M62.087 E41M47.150 GWM165.172 E41M47.301 E41M62.091 E41M49.343 E41M62.246 E36M48.272 BCD250 E36M61.332 P33M47.142 PRC140.409 P42M61.182 P33M60.110 E38M60.146 E41M50.330 E36M61.306 E38M47.284 E38M47.363 E41M61.317 E41M59.230 E36M59.113 E36M49.238 E37M60.135 E38M47.068 E37M49.273 KSU149.240 E38M49.170 E38M49.291 E37M47.108 E36M50.171 E36M62.341 E36M48.118 E36M61.099 E41M62.369 E36M62.087 P35M50.262 E41M62.246 E36M61.332 E41M61.317 P35M62.123 P33M60.110 E38M60.146 E36M61.306 E38M47.284 E41M59.230 E36M59.113 E36M49.238 E41M61.340 PRC140.409 KSU149.243 E37M47.108 E38M49.291 E36M48.118 4H 4D E36M48.277 E41M47.171 P33M61.340 E41M47.172 E38M60.201 KNOX.239 4H E38M59.100a E41M47.060 E38M60.396 4H TCP1Xm.459 TCP1Xm.459 4H E38M60.196 4H KNOX.239 E41M61.269 E36M49.076 E38M59.100a E41M47.060 P33M62.206 E41M61.269 E36M49.076 E37M60.190 E37M60.090 P44M49.326 P35M49.205 P35M60.313 P35M62.276 P35M62.274 100 105 E36M50.064 P35M60.392 P35M50.371 P35M50.372 P42M61.285 E37M62.368 E41M62.082 E41M62.233 P44M49.141 P33M48.359 E41M62.200 P42M62.362 E37M49.360 P44M62.317 4 E38M47.398 E41M49.223 E41M49.283 E41M61.172 P35M62.140 P42M61.073 E41M49.277 E36M59.152 P42M62.263 E41M62.219b 3 E36M61.368 2 P33M61.316 E36M61.289b 35 45 E38M59.239 1 30 P44M62.317 P44M62.331a E38M60.091 4H MipsA.284 4B KSU154.137 E41M62.396 4B KSU154.137 E36M62.210 P33M59.260 0 25 E38M60.090 P33M62.351 E36M50.064 E37M49.207 E37M49.206 P33M59.134 4BH GWM006.170 P33M48.399 E41M49.194 E36M48.109 E37M62.129 E37M62.128 P35M61.153 4H MLOH1A.352 P35M59.230 E41M47.169 LG4Xm--TTC2 6 5 4 3 2 1 0 20 LG4Xm--TTC1 6 5 4 3 2 1 0 15 4 10 3 5 LG4Ns--TTC2 2 P33M59.260 P35M62.140 E41M61.172 E41M49.283 E41M49.223 E36M62.349 P42M61.073 P44M49.095a E41M49.277 E37M47.143a 1 0 E38M59.239 0 P33M47.125 E38M47.198 E41M48.093 P35M49.201 P33M61.273 P35M62.276 P33M47.126 P35M62.274 110 E36M62.211 115 120 P35M62.127 TCP1Ns.430 125 E36M62.211 130 4H E38M49.390 E38M60.223 E37M60.252 E41M61.380 E41M47.384 E36M59.247 TCP1Ns.430 E37M60.375 P35M62.127 135 140 145 P33M61.096 E41M60.301 E36M49.279 E41M48.195 E38M49.390 E36M59.247 E41M61.212 E41M61.272 4H CIRC_IM BOLT_IM CIRC_rMQM BOLT_rMQM ANTH_IM HGHT_IM ANTH_rMQM HGHT_rMQM LG5Ns--TTC1 LG5Ns--TTC2 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 45 5B 50 55 E36M62.340 P33M62.259 60 E36M59.111 65 E36M48.254 P33M50.232 70 E36M62.189 75 80 85 E41M62.107 E37M60.105 E41M61.201 E41M61.256 E36M61.139 E37M47.322 5ABD E37M61.149 E41M60.285 E41M61.136a E37M47.133 E41M60.293b E36M61.383 E41M61.091b E36M48.180 E38M49.222 E37M62.136 E38M49.369 E37M47.378 P33M60.366 KSU171.279 P33M50.340 P33M50.158 E38M59.268 E41M49.140 E37M62.273 E36M62.311 E38M60.107b E41M47.281 E36M48.296 E38M60.169 P33M50.233 E36M48.308 E36M62.189 E36M48.254 E41M62.107 E41M59.267 E41M61.201 E41M61.256 5B 5ABDHR PSR128.412 P35M62.264 E36M61.173 E41M48.173 E41M48.103 E36M48.088 E41M59.130 95 100 E37M60.307 E37M61.327 E37M60.092 E41M48.103 5ABDHR CDO504Xm.167 E37M47.096 E37M47.136 E37M47.324 E38M59.199 E37M60.105 E37M49.183 E36M49.188 110 115 E36M50.333 E36M62.180 P33M59.264 P35M61.295 P33M62.323 E41M61.194 E38M47.084 E41M60.244 E38M59.248 E41M50.064 P33M62.172 P35M60.115 P33M62.178 E41M60.140 E41M59.311 125 E41M59.312 E36M59.140 E41M60.099 E36M59.265 130 E37M47.153 E41M48.056b E37M47.244 P42M62.112 135 E37M60.235 P33M60.077 P42M61.143 P33M48.283 140 P33M48.258 P35M49.344 4H-5A VRN2.199 P33M50.292 145 E36M49.319 E36M50.319 E36M48.207 E41M61.279 150 120 CDO504Xm.167 5ABDHR E36M62.220 P35M59.137 E41M60.229 E37M49.077 P35M59.102 E36M48.200 P44M62.088b E41M49.213 P44M62.089 P35M60.240 E37M61.215 E41M62.122 P33M59.262 P35M62.167 P35M60.100 P33M47.216 E36M62.252 E41M60.147 P33M48.361 E36M62.159 E36M50.333 E36M49.333 E37M61.190 E41M59.303 E37M49.100 E37M47.063 E37M49.063 E36M61.125 P35M59.137 E41M49.213 E41M62.122 P44M62.088b P44M62.089 E36M62.334a E41M60.058 P35M60.240 P35M60.239 P33M48.153 CDO504Ns.086 5ABDHR P35M62.167 E38M47.084 E37M60.200a E36M49.138 P44M62.296 P35M60.100 P33M47.216 E41M48.075 E37M62.155 E37M49.100 P35M49.065b P33M62.335 E41M60.244 E41M60.079 E38M59.248 P33M62.172 E36M59.265 P35M60.115 E37M49.352 E41M48.056a E38M59.149 E41M48.056b P42M62.112 E36M48.158 155 P33M62.208 E37M61.190 E36M59.312 P35M49.251 105 5ABD E41M48.119a E36M62.180 P33M59.264 P42M62.191 E38M49.230 E41M47.149 P33M47.211 E38M47.338 E36M59.291 P33M59.104 E37M62.120 5H MWG2230.285 P35M59.376 P33M48.340 5ABD(F) (F) 7B E38M59.199 E41M59.199 90 5B 5H E37M47.244 P33M60.077 CIRC_IM ANTH_IM CIRC_rMQM ANTH_rMQM BOLT_IM HGHT_IM BOLT_rMQM HGHT_rMQM 160 165 P42M61.139 E37M60.235 P35M49.344 P33M50.292 VRN2.193 P44M49.296 4H-5A 4 40 E38M60.094 5AD 3 35 E37M47.133 E38M60.327 E41M61.091b E36M61.339b E36M48.180 E36M59.225 P33M60.366 E37M60.287 E36M61.383 E36M62.311 E38M59.268 E37M61.204 E37M60.398 KSU171.279 P33M50.158 E41M48.280 E37M47.378 E38M60.107b E41M47.281 2 E37M61.149 E41M60.357 30 1 E38M60.094 25 E41M59.333 E36M49.272 E41M48.244a E36M59.308 P33M61.089 E37M61.269 E36M61.218 E37M49.073 E41M47.364 E37M60.156 E41M49.094 P35M61.131 E41M59.260 E41M49.120 P33M59.200 GWM205.142 E37M49.250 P33M59.169 GDM101.197 P33M59.170 P35M61.102 TCP2Xm.252 E41M49.333 E38M60.204 E38M47.124 E38M47.147 E38M49.245 E38M47.313 E41M60.205 E37M60.172a E36M59.133 BCD1130 BCD1707 E36M50.284 KSU220.308 E36M62.330 E36M49.284 E41M48.059 P35M50.363 E41M60.163 P33M61.250 E38M59.139 P44M49.085 P42M61.257 E37M47.307 E36M62.325 P33M50.244 E38M49.191 E41M49.192 GDM068.116 E41M60.267 P33M48.298 E38M49.207b 0 P33M50.274 E38M60.248 E37M60.228 E37M62.163 20 4 P42M62.381 GDM101.196 E38M59.137 E41M59.333 E41M48.244a E36M59.308 P42M61.162 P33M61.123 P33M59.169 E41M49.094 E37M60.156 E41M61.224 E37M49.251 E41M49.120 E41M59.114a E38M49.136 E36M50.284 E41M48.336 E41M47.258 E38M47.147 P35M50.363 E36M49.284 E38M60.204 E38M47.313 E41M60.205 E36M61.082 E37M60.172a E38M49.245 E41M60.164 GDM068.114 P33M61.250 E37M61.291 E38M49.191 P44M49.085 P33M50.244 E37M47.341 E41M60.276 E38M59.139 E41M49.191 E38M60.178 P42M61.100 P33M48.298 E36M61.352 E41M48.062b P35M49.199 P33M50.197 3 5H P35M50.281 P33M62.133 P35M62.390 E36M48.290 LG5Xm--TTC2 2 5B 1 0 4 15 3 5H 2 10 P33M60.202 P42M62.081 P35M62.389 TCP2Ns.117 P33M62.093 P33M62.097 E36M59.169 E37M62.172 1 P33M62.175 E41M48.077 E37M62.172 TCP2Ns.117 5 LG5Xm--TTC1 0 4 3 2 1 0 P33M62.133 0 LG6a--TTC1 LG6a--TTC2 E38M47.183 P33M48.087 P35M61.198 P44M62.219 P33M61.168 E37M47.093 E41M61.134 E41M48.235 E37M49.233 P33M61.280 E36M62.114 E38M59.319 P33M48.296 P44M62.200 E36M59.260 P33M48.087 P35M61.198 P44M62.219 P33M61.168 E41M61.134 E37M47.093 E37M60.158 E36M62.114 E41M59.185 E38M59.321 E36M62.374 P44M62.200 E41M47.397 P44M49.151 P33M60.356 E37M62.189 P35M49.269 P33M61.149b P33M48.131 E37M47.083 E38M60.144 P35M49.164 80 E36M62.292 E36M62.177 E41M47.385 P42M62.269 E41M62.076 E38M49.336 E36M49.310 E38M59.160 P33M48.386 P35M62.209 P42M62.293a P42M61.099 E41M61.399 E36M59.245 E38M59.291 85 90 95 E36M61.170 E38M47.157 P35M50.389 E41M47.205a E41M61.259 E41M59.353 P33M60.252 P35M62.313 P35M62.344 E37M61.282 E36M48.329 E41M48.079a E41M62.150 E41M48.205 P35M62.205 P33M47.170 P33M47.206 E41M47.385 60 P35M60.093 E36M61.112 P35M62.312 E36M48.329 P35M60.106 P44M62.196 55 E41M60.287 E41M61.276 P35M60.093 P33M48.131 E36M61.344 75 4 6B E41M60.122 E41M47.350 70 3 P35M62.066 P33M60.154 P44M49.121 P33M62.219 P33M47.398 P42M62.206 E37M61.063 6H MWG652.264 P35M49.196 P35M49.194 6B GIRi5.351 P44M49.065 P33M50.323 6H 45 65 2 P35M50.213b P33M50.323 P44M49.345 50 1 E38M49.197 E38M49.103 E41M49.122a E37M49.135 E36M48.257a P44M49.121 P42M62.205 MWG652.264 P33M62.219 P44M49.205 GIRi7.394 P35M59.091 E37M61.063 E37M60.089 E41M62.334 E41M49.246 E41M62.279 P33M60.203 E41M47.350 40 0 4 3 2 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. LG6b--TTC2 P35M50.213b 30 35 1 0 6 5 4 3 25 2 1 20 0 15 LG6b--TTC1 P35M62.193 P33M60.214 5 10 4 P35M62.193 3 2 1 0 0 P35M62.344 P33M47.133 P35M62.183 E36M62.178a P35M62.209 E38M49.336 MWG2264.403 6H E36M49.310 E41M62.113 E41M62.076 E38M47.252 E38M59.161 E36M62.292 E37M47.346 P35M62.251 P33M47.206 P33M47.132 P42M61.099 P33M50.278 E38M59.291 E36M61.170 E38M47.157 E41M61.259 P33M60.252 E41M60.363 E38M60.205 1R E41M60.098b E41M47.058 P35M62.183 E36M61.255 E36M48.196a E41M60.098a P42M62.163 E37M62.190 E38M59.264 E41M61.105 E36M61.252 E38M49.315 P33M60.310 E41M61.098 E41M62.313 E36M48.327 E41M60.098a E36M61.255 E37M49.363 TRX2 E36M62.118 E41M62.157 E36M49.274 E37M62.190 P35M60.149 E36M61.252 E37M49.363 TRX2 E38M49.315 P35M60.149 E41M60.363 100 1R E37M61.133 E41M47.099 7D GDM150.365 E41M60.232 P33M50.258 P42M62.284 P35M50.236 E37M60.290 E41M61.192 P33M60.390 P44M49.312 P44M62.284 P35M61.301 E37M47.366 P35M59.243 P42M61.319 P35M49.381 P35M49.378 P33M62.108 E41M59.252 E37M62.268 E37M60.328 E36M62.219 P35M62.223 105 110 P35M62.224 P35M61.164 P33M62.217 115 P35M60.169 120 P33M62.213 E41M62.185 125 130 135 140 145 E41M62.185 E41M59.349 E37M62.371 P44M62.103 E38M59.286 E38M49.278 E41M59.367 P33M59.267 P35M59.214 P35M50.093 P35M61.205 P35M59.093 E38M47.329 E36M48.152 E37M47.102 E41M59.348 E41M59.368b P44M62.103 E38M49.278 E38M47.353 E36M48.196b P33M59.273 E37M61.133 E41M47.099 P42M62.284 P33M50.258 GDM150.365 7D E41M60.232 P35M50.236 E36M49.295 E41M61.192 P35M49.378 E37M62.268 P35M59.243 P35M49.381 E37M47.366 E37M60.356b E41M59.291 E41M59.313 P44M62.284 P44M49.070 P33M62.313 P35M62.100 E38M60.334 E36M62.219 P35M59.214 P35M50.093 P35M61.205 E41M60.071 E41M60.071 E36M61.367 P35M60.286 CIRC_IM BOLT_IM ANTH_IM HGHT_IM CIRC_rMQM BOLT_rMQM ANTH_rMQM HGHT_rMQM LG7a--TTC1 E41M59.278 E41M60.070 40 P44M62.197b P42M62.326 E38M60.140 45 E37M62.297 50 55 7H 60 7H(D) 65 6ABDHR 5A,5B,5D 5A,5B,5D 70 75 80 85 90 E41M49.357 E41M49.170 E41M47.162 E36M62.306 MWG741.287 E41M62.351 BCD421 E36M62.225 E37M62.323 MWG669.492 ADP.350 P42M62.289 ADP.190 E38M49.297 E41M48.294 E36M59.391 E36M62.298 E36M49.380 E38M60.092 E41M61.112 E41M50.267 P33M62.126 P33M47.242 E36M59.279 E41M60.338 E37M61.340 E41M62.342 E41M62.103 E38M59.259 E41M47.066 E41M61.313b E41M47.162 E38M59.342 E36M61.067 E36M59.263 E41M60.336b MWG741.287 7H E38M49.136 E36M61.122A E36M62.225 E37M62.323 E41M61.111b E41M62.116 P44M62.226 E38M49.297 E41M60.239 E38M60.092 E41M61.267 E41M48.294 E41M61.112 E37M61.340 E41M47.220 E36M49.380 E41M60.338 E41M59.114b P44M62.288b MWG669.492 6ABDHR E37M47.259 E41M60.378 P44M62.063 P44M49.129b E36M50.314 E38M59.300 P33M48.319b P42M61.322 P44M49.130 E36M50.202 95 4 E37M49.142 E37M49.143 E38M60.140 3 35 2 E36M61.152b E37M49.198 E41M49.193 P33M61.326 1 30 0 P33M48.240 4 E38M60.148 3 P44M62.197b E37M49.382 E41M48.254 LG7b--TTC2 2 E41M62.212 P35M50.219 1 7H P33M50.143 P33M48.159 E41M48.215 E37M49.340 E41M48.153 MWG703.194 7H E36M62.088 E36M48.336 P44M62.397 E41M62.212 E41M49.259 E41M48.162a P35M50.101 E41M59.215 E36M59.095 E36M49.090 E41M61.084a E37M62.291 E37M62.071 P33M61.387 7H 6-SFT.579 E41M59.072 P44M49.179 P33M50.284 P35M59.166 P33M48.135 P35M61.154 E38M47.385 P33M62.071 E41M60.130 P33M62.352 E38M47.222b 0 6-SFT.101 4 P33M47.119 3 P44M62.094 2 1 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. LG7b--TTC1 0 25 4 20 3 15 LG7a--TTC2 2 10 1 5 E36M49.124 P33M50.361 E38M49.207a E38M60.308 P33M59.082 P44M62.094 7H 6-SFT.101 P33M50.267 E36M62.193 E41M48.215 E41M48.152 7H MWG703.194 P35M49.204 E41M49.204 E36M61.095 E36M49.061 P33M61.386 0 0 E41M59.215 E41M60.235 6-SFT.579 7H E36M59.100 E36M62.261 E36M61.316 E37M62.291 E41M61.084a E38M59.323 P44M49.228b P33M50.284 E37M61.205 E41M59.072 P35M62.184 P35M59.165 P33M62.071 E38M47.385 E41M60.130 P44M62.117 E36M48.130 E37M61.193 E37M47.078 E41M61.091a P35M60.246 E36M59.371 E38M59.304 P35M60.247 LIMDEX.227 7H P35M59.116 P42M62.386 P33M50.349 E38M59.298b E37M61.193 E41M47.266 E38M49.375 E41M61.090b E41M61.091a P35M60.247 E36M59.134 E38M59.304 E41M59.234 P35M60.189 P35M59.116 E37M62.180 E36M49.190 E38M47.096 P44M49.261 P33M48.384 E37M62.180 E41M60.190 E38M47.096 P35M62.109 P33M62.325 E41M47.170 P33M62.325 P35M62.254 P33M48.319a E36M61.093 E38M59.082 E41M48.321 E41M48.111 E36M61.289a E36M49.327 E38M60.165 E38M47.171 E37M60.144 E37M47.082 P33M61.368 P33M62.327 P44M62.290 E37M47.222 E41M47.134 E37M60.148 E38M59.082 E41M48.111 E41M59.095 E37M49.380 E37M62.157 E37M60.144 E41M59.203 E41M48.355 E36M62.334b P44M62.290 E41M59.203 P35M49.347 P35M49.347 P35M61.311 E36M61.320 E38M47.384 E41M48.338 P35M61.382 7A WMC488.105 P33M62.122 E36M61.122 WMC488.105 7A E41M48.240 E41M59.273 100 E41M60.378 105 110 P44M49.129b E37M60.246 E36M49.258 E36M50.202 115 E41M62.362 P44M49.130 120 125 130 135 140 145 E36M62.325 E36M59.237 E37M60.107 E41M47.310 E36M61.142 E37M61.374 P33M48.294 P33M50.156 P33M50.142 P35M62.166 E37M47.169 E36M49.270 E38M47.230 P33M48.174 P35M62.280 P33M60.318 P33M48.178 E41M47.257 E37M60.106 E37M60.092 E38M59.327 P35M50.209 P35M62.166 E37M61.374 E36M62.300 E41M62.287 E41M59.252 E37M47.294 P33M60.318 P33M48.174 E41M61.090a E36M49.269 P35M62.280 P33M48.178 E41M47.257 E41M49.313 E41M60.185 E41M47.313 E38M47.261 E41M49.065 E37M62.230 E36M50.212 P33M47.108 E41M61.262 E41M61.240 E37M61.225 E37M61.283 E41M59.220 P33M48.122 P33M47.230 E41M62.229 P33M60.378 P33M50.332 P35M61.183 E36M49.368 E37M49.356 E37M60.323 E41M47.399 E38M59.151 E36M62.383 E36M50.325 E36M62.172 P33M62.181 E36M48.313 P35M50.285 P35M61.381 E37M49.108 P35M59.237 P33M59.186 E38M49.305 P35M61.311 E36M62.172 P33M48.122 P44M62.156 E41M62.229 E41M48.377 E36M49.101 E36M50.398 E37M49.113 E41M47.255 P33M60.378 E36M48.293 E36M50.325 E36M50.269 P35M62.095 E41M48.384 P33M61.074 E38M49.305 E36M48.313 P35M61.143 P35M61.381 P33M60.225 E41M60.185 150 155 P33M61.074 CIRC_IM BOLT_IM ANTH_IM HGHT_IM CIRC_rMQM BOLT_rMQM ANTH_rMQM HGHT_rMQM Histograms for circumference (cm) of plant spreading in the full-sib Leymus TTC1 (n=164) and TTC2 (n=170) mapping families, based on means of two clones, compared to the heterogeneous L. cinereus Acc:636 (Lc) and L. triticoides Acc:641 (Lt) progenitors, interspecific hybrid parents (TC1 and TC2), and recurrent parent (T). 60 55 2002 ID Lc Lt T TC1 TC2 TTC1 TTC2 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 50 45 40 35 30 AV (SD) 23 (10) 354 (186) 487 (61) 96 (35) 125 (37) 180 (91) 299 (116) TTC1 TTC2 25 20 15 10 5 0 70 Lc 140 210 280 350 TC1 TC2 TTC1 TTC2 420 Lt 490 560 630 700 770 840 910 980 1050 More T 50 45 2003 ID Lc Lt T TC1 TC2 TTC1 TTC2 40 35 30 25 AV (SD) 51 (26) 594 (326) 853 (120) 200 (32) 231 (38) 288 (122) 449 (154) TTC1 TTC2 20 15 10 5 0 70 140 Lc 210 280 350 420 TC1 TC2 TTC1 490 560 TTC2 630 700 770 840 Lt 980 1050 More T 40 35 910 ID Lc Lt T TC1 TC2 TTC1 TTC2 2004 30 25 20 AV (SD) 76 (21) 816 (416) 1263 (177) 256 (39) 282 (53) 362 (137) 548 (170) TTC1 TTC2 15 10 5 0 70 140 Lc 210 280 350 420 TC1 TC2 TTC1 490 560 630 TTC2 700 770 840 Lt 910 980 1050 More T Histograms for proportion of bolting culms in the full-sib Leymus TTC1 (n=164) and TTC2 (n=170) mapping populations, based on means of two clones, compared to the heterogeneous L. cinereus Acc:636 (Lc) and L. triticoides Acc:641 (Lt) progenitors, interspecific hybrid parents (TC1 and TC2), and recurrent parent (T). 110 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 100 ID Lc Lt T TC1 TC2 TTC1 TTC2 2002 90 80 70 60 AV (SD) 0.28 (0.46) 0.67 (0.48) 1.00 (0.00) 0.79 (0.43) 1.00 (0.00) 0.57 (0.43) 0.72 (0.39) TTC1 50 TTC2 40 30 20 10 0 0.1 0.2 0.3 0.4 0.5 Lc 0.6 0.7 TTC1 Lt 0.8 0.9 1 T TC2 TTC2 TC1 40 35 2003 ID Lc Lt T TC1 TC2 TTC1 TTC2 30 25 20 AV (SD) 0.63 (0.43) 0.56 (0.32) 0.98 (0.06) 0.88 (0.19) 0.77 (0.21) 0.33 (0.24) 0.55 (0.26) TTC1 TTC2 15 10 5 0 0.1 0.2 0.3 0.4 0.5 TTC1 0.6 TTC2 Lt 0.7 Lc 0.8 TC2 0.9 1 TC1 T 40 35 2004 30 25 20 ID Lc Lt T TC1 TC2 TTC1 TTC2 AV (SD) 0.85 (0.33) 0.78 (0.35) 1.00 (0.00) 0.84 (0.19) 0.81 (0.23) 0.55 (0.26) 0.67 (0.27) TTC1 TTC2 15 10 5 0 0.1 0.2 0.3 0.4 0.5 0.6 TTC1 0.7 TTC2 0.8 0.9 Lt TC2 TC1 Lc 1 T Histograms for anthesis date (days from January 1) in the full-sib Leymus TTC1 (n=164) and TTC2 (n=170) mapping populations, based on means of two clones, compared to the heterogeneous L. cinereus Acc:636 (Lc) and L. triticoides Acc:641 (Lt) progenitors, interspecific hybrid parents (TC1 and TC2), and recurrent parent (T). 100 90 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 80 ID Lc Lt T TC1 TC2 TTC1 TTC2 2002 70 60 50 AV (SD) 176.8 (2.1) 173.3 (2.6) 172.0 (0.0) 173.6 (2.1) 173.1 (1.5) 174.8 (1.9) 173.8 (4.9) TTC1 TTC2 40 30 20 10 0 166 169 175 178 181 184 187 190 193 T-tester Lt TTC2 Lc TC2 TC1 TTC1 55 50 172 ID Lc Lt T TC1 TC2 TTC1 TTC2 2003 45 40 35 30 AV (SD) 171.1 (5.1) 169.3 (1.4) 169.0 (0.0) 169.0 (0.0) 169.0 (0.0) 172.1 (5.7) 172.1 (5.5) 25 TTC1 TTC2 20 15 10 5 0 166 169 172 175 178 181 184 187 190 193 T-tester Lt Lc TTC1 TC1 TC2 TTC2 70 65 60 55 50 45 40 35 30 25 20 15 10 5 0 ID Lc Lt T TC1 TC2 TTC1 TTC2 2004 AV (SD) 178.3 (3.9) 172.6 (1.6) 173.0 (0.0) 175.9 (4.0) 175.9 (4.0) 175.7 (4.6) 174.2 (4.0) TTC1 TTC2 166 169 172 175 178 Lt TTC2 TC1 T-tester TC2 181 Lc 184 187 190 193 Histograms for plant height (cm) in the full-sib Leymus TTC1 (n=164) and TTC2 (n=170) mapping populations, based on means of two clones, compared to the heterogeneous Leymus cinereus Acc:636 (Lc) and L. triticoides Acc:641 (Lt) progenitors, interspecific hybrid parents (TC1 and TC2), and recurrent parent (T). 50 Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. 2002 ID Lc Lt T TC1 TC2 TTC1 TTC2 40 30 AV (SD) 99.0 (39.8) 94.5 (12.6) 100.8 (5.7) 145.8 (15.9) 141.8 (10.6) 103.9 (13.8) 109.7 (13.0) TTC1 TTC2 20 10 0 60 70 80 90 100 110 120 130 140 Lt Lc T TTC1 TTC2 150 160 170 180 TC2 TC1 70 ID Lc Lt T TC1 TC2 TTC1 TTC2 2003 60 50 40 AV (SD) 135.6 (23.0) 98.5 (8.5) 100.9 (5.9) 155.1 (12.6) 138.4 (13.3) 108.3 (12.9) 109.7 (13.0) TTC1 TTC2 30 20 10 0 60 70 80 90 100 110 Lt T 120 130 TTC1 TTC2 140 150 Lc TC2 160 170 180 TC1 60 ID Lc Lt T TC1 TC2 TTC1 TTC2 2004 50 40 30 AV (SD) 182.9 (18.5) 123.3 (12.7) 125.7 (3.4) 184.7 (12.3) 164.6 (8.7) 134.8 (13.8) 137.8 (11.8) TTC1 TTC2 20 10 0 60 70 80 90 100 110 120 130 140 150 Lt T TTC1 TTC2 160 170 TC2 180 Lc TC1 Description of additional SSR (CNL and KSU) and STS markers added to the Leymus TTC1 and TTC2 maps originally published by WU et al. (2003) Loci(groups) Primers Reproduced from Crop Science. Published by Crop Science Society of America. All copyrights reserved. TRX1 (LG1a, LG2b) GGATAACATGACCCTAAAAACT† GTTGTGCATCACAGGGTTATA TRX2 (LG6B) GGATAACATGACCCTAAAAAG† TTGTTCATCACAGGGTTATC CNL45 GAGAGAGCTTCGTCCCACTC CACCACTCGTTTCTTTCACTTT (LG2A, LG2B) KSU154 (LG4Ns) CNL39 (LG4Ns) BCD1117 (LG4Ns, LG4Xm) HVCABG (LG4Ns) HVM068 (LG4Xm) KSU149 (LG4Xm) KSU171 (LG5Ns) MWG2230 (LG5Ns) VRN2 (LG5Ns) MWG2264 (LG6a) ADP (LG7a) GGAGACTCTGGTCATCTCGC ATACTGGAGTGAAGGCACGG TACCTGTGCGGCGATGAAT CAGGAGCAGGAGAACGTGAA TCAGTTCTCAATAGAAGTGCTGTG TCCTGAATAAGGTCTTCATACCAA ACACCTTCCCAGGACAATCC CAGAGCACCGAAAAAGTCTGTA AGGACCGGATGTTCATAACG CAAATCTTCCAGCGAGGCT GAGCCACCAGAGCAGAAATC CGAGCTCCCCTTCTTCTTCT TCTTGCTTGCATTGTAACCG TCATGTCTGGGAGCATGGTA AATGATGTTGCTTTCCTGTTTGCTC ACAGATGATGATGGCGTGCAGCTTT AGTACCAGTTCTTCRCCCAAGG CTGCASYAGGTGAGCCAT AGGTAGAAGTCAAACTGTGTGGGAT GTATTACTTTACGAGTTAGATGCTA CCTCCGTGAACAATTTCCTG TCCAATACGAGCATTCTTGT Gene or motif Anchor loci Expected amplicon Mapped amplicons§ AY204511 thioredoxin, Xbm2, 1R 287‡ Leymus 289 AY204511 thioredoxin, Xbm2 1R 287‡ Leymus 288 (ga)9, putative RNA binding protein 2A 167‡ wheat 154, 161, 170, 172 (cac)7 4B 146‡ wheat 137 AF251264 (atgc)5 rubisco activase B 3A 220‡ wheat 229 BCD117 barley cDNA clone 4HD 209 152, 211 Rubisco (AT)29 4H 182 barley 152 (GA)22 4H 204 barley 199 (acc)9 4D 228‡ wheat 240, 243 (agt)7 5B 243‡ wheat 279 MWG2230 barley genomic DNA 5H 320 barley 285 Wheat vrn2 (AY485644) 4H, 4A/5A 203 wheat 193,199 MWG2264 barley genomic DNA 6H 400-450 403 1003‡ rice 350 TaqI 190 TaqI M31616 ADP glucose phosphorylase 5ABD † FAM 5’ labeled primer. ‡ Amplicon size based on sequence data. § Amplicon size based on comparisons with internal size standards in capillary electrophoresis.