Ebola Vaccine Development Sarah Gilbert, Jenner Institute, University of Oxford
advertisement
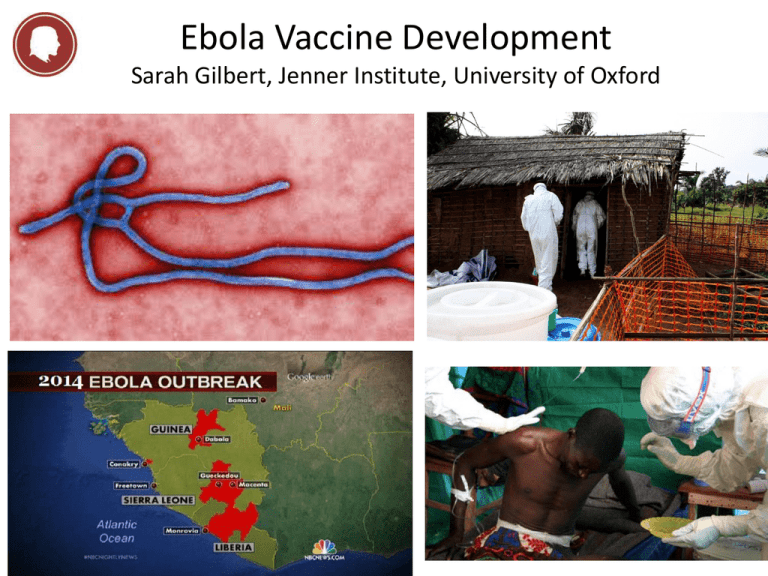
Ebola Vaccine Development Sarah Gilbert, Jenner Institute, University of Oxford THE JENNER INSTITUTE THE JENNER INSTITUTE a partnership between Oxford University and the Pirbright Institute - Developing innovative vaccines - Partnering with industry - Driving the One Health agenda The Jenner Institute • Global Health – vaccines that make a difference • HIV, TB, malaria, dengue, pandemic influenza • Translational Research – rapid early clinical testing • 32 vaccines made for clinical trials • One Health – vaccines for humans and other animals Human Vaccines Pipeline Disease Area Number of GMP Vaccines Preclinical Phase I Phase IIa Oxford Malaria 15 TB 3 HCV 3 HIV 5 Pandemic Flu 2 Meningitis 1 RSV 3 Ebola 1 Phase Ib Phase IIb Phase III Patient Group /Endemic Area Staph aureus Prostate cancer Rift Valley Fever The busiest pipeline of any non-profit vaccine institute Licensure Ebola in West Africa, 2014 • Epidemic worsening in Guinea, Liberia, Sierra Leone – international public health emergency – >13 567 cases, >4 951 deaths (by 31st October) – No vaccines, no drugs licensed • Two vaccines show single dose efficacy in macaques – Chimpanzee adenovirus – Vesicular stomatitis virus • Both vaccines encode the surface glycoprotein – 5 major strains defined: Zaire and Sudan most common – Guinea outbreak is the Zaire strain Pre-clinical Ebola Vaccine Development • Nancy Sullivan group NIH, vaccine development with Ebola glycoprotein in non-human primates • 2000: DNA prime, replication deficient Ad5 boost (1010 pfu) – All vaccinated animals asymptomatic after challenge • 2005 recombinant attenuated VSV (replication competent) – Single dose 5 x 107 pfu was completely protective • 2006 single dose Ad5, (1010 vp = approx 108 pfu) – Complete protection 4 weeks after immunisation Clinical Ebola Vaccine Development • Nancy Sullivan group NIH, vaccine development with Ebola glycoprotein in clinical studies (Zaire and Sudan together) • 2006: DNA vaccine is safe and immunogenic – – – – 3 doses of either 2, 4 or 8 mg DNA vaccine Safety good ELISA and CD4+ responses detected in 20/20 volunteers CD8+ responses in 6/20 volunteers • 2010 replication-deficient Ad5 vectored vaccine is safe and immunogenic – – – – – 2 x 109 or 2 x 1010 vp single intramuscular injection Safety good 50% of volunteers developed antibody responses 4 weeks after immunisation Roughly 50% of volunteers developed CD4+ responses Those with pre-existing neutralising antibodies to Ad5 made less response More Pre-clinical Ebola Vaccine Development • Nancy Sullivan group NIH, vaccine development with Ebola glycoprotein in non-human primates • Ad26 in NHPs with pre-existing immunity to Ad5 – Partial protection from Ad26 • No survival when 1010 vp used • 50% survival when 1011 vp used • 75% survival when 1012 vp used – Complete protection when boosted by Ad35 • 1011 vp of each vaccine 4 weeks apart • 100% survival after challenge • ChAd3 completely protective at 1010 or 1011 vp – Short term protection, challenge 5 weeks after vaccination – When challenged 6 months after vaccination, 0 or 50% protection – Boost with 1 x 108 pfu MVA after 8 weeks, complete protection when challenged 10 months later Accelerated Ebola Vaccine Development • Chimpanzee adenovirus 3 vaccine chosen – Previously used for hepatitis C phase I trials • WHO / Oxford / Wellcome / GSK / Okairos / NIH plan – – – – Phase I in Oxford mid-September: 60 volunteers Phase I in Mali: 80 volunteers Phase I in Switzerland Parallel manufacturing of 10,000 doses of ChAd3 EBO Z • Objectives – Safety data in 140 volunteers – Immunogenicity comparable to protected macaques • Decision on whether to deploy: December 2014 – Primary target population: healthcare workers Accelerated Ebola Vaccine Development • Attenuated VSV, replication competent • VSV-Zebov is based in part on a genetically engineered version of vesicular stomatitis virus , which primarily affects rodents, cattle, swine and horses. • Human VSV infections are rare and generally produce three to four days of mild illness • Has been given post exposure to two accidentally infected lab workers – 5 x 107 pfu, resulted in fever • Otherwise no experience with the vector in clinical trials – Virus shedding, extent of replication and persistence unknown • Long term protection in rodents 12-18 months after single dose of 2 x 105 pfu • Trials will test 106 to 108 pfu – 800 vials delivered to WHO – One or two doses to be tested in US – More trials in Switzerland, Germany, Gabon, Kenya Process Development Challenges mitigated by using platform technologies • Which vaccine? – ChAd3, VSV, Ad26, MVA – Virus-like particles • What dose? – ChAd3 (1 x1010 vp), 2.5 x1010 vp 5 x1010 vp – VSV 106 to 108 pfu • How many doses per person? – One or two • How many vaccines? – Ad prime MVA boost • How many people? – Front line workers – Population in isolated areas where new outbreaks occur Expected numbers of doses • VSV, depends on dose – between 120,000 and 12 million doses available next year • ChAd3 – Original batch size of 10,000 doses, can expand capacity • MVA – Process for thousands of doses exists • Sterile filling capacity will be needed in all cases • WHO asking for 500,000 doses by mid 2015, plus another 500,000 by end 2015 Testing Efficacy and Defining Correlates of Protection • Can and should efficacy be tested? • Has implications for outbreak control, defining CoP, defining optimum dose/regimen, best use of available funds • Stepped wedge or randomised placebo controlled trial • CoP – Total IgG – Neutralising antibodies – T cell response • Duration of immunity – Requirements depend on intended use Does it Have to Be This Way? “Well, that’s another fine mess…!” Rapid Response versus Outbreak Preparedness Some Outbreak Pathogens • Rift valley fever virus • Ebola virus • Chikungunya virus • Marburg virus • MERS coronavirus • Pandemic influenza • Crimean-Congo hemorrhagic fever virus • Enterovirus 71 • Hendra virus • Lassa virus • Monkeypox virus • Nipah virus • SARS coronavirus • Venezuelan equine encephalitis virus • West Nile virus Figure S1: Vaccine design and confirmation of transgene expression A Vaccine design using ChAdOx1 and HAdV5 Human tissue plasminogen activator leader sequence V5 tag MP-12 RVF virus GnGc Human cytomegalovirus major immediate early promoter ChAdOx1 or HAdV5 genome Pb9 tag BGH poly (A) tail ChAdOx1 or HAdV5 genome B 75KDa Anti-Gn antibody 50KDa 75KDa Anti-Gc antibody 50KDa Warimwe et al. Virol J. 2013;10:349. Immunogenicity and efficacy against RVF viral challenge in mice 8 weeks post-vaccination 1000 VNT50 ** ** 100 100% protective efficacy by all regimens in BALB/c mice ChAdOx1-GnGc +/- Matrix-Q™ selected for ruminant trials x1 dO hA C C hA dO x1 -G C hA dO nG x1 c -G -G + A nG d d n Gc c aV + M ax ™ at rix HA -M dV ™ 5H H Ad A d Gn G V5 V5 c -G -G + A nG dd nGc c aV + M ax™ at r ix -M ™ 10 Warimwe et al. Virol J. 2013;10:349. Evaluation of ChAdOx1-GnGc in ruminants • Species: Sheep, cattle, goats (locally available breeds) • Regimen: Single dose (109 infectious units) intramuscularly • RVF viral challenge: 4 weeks post-vaccination • Efficacy endpoints: Fever, viraemia, monitored over 2 weeks A Suggested Way Forward for Outbreak Pathogens • Vaccine development to phase II trials for all of these pathogens – Safety and immunogenicity as for Ebola – Largely public funding – Preferably a common manufacturing platform • Vaccine stockpiles held in affected regions – 10,000 to 50,000 doses – Emergency use approvals and efficacy evaluation – Learn from existing stockpiling strategies




