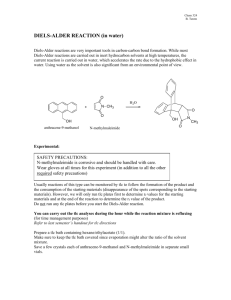
Diels-Alder Reaction Lab Notes: Synthesis & Safety
advertisement
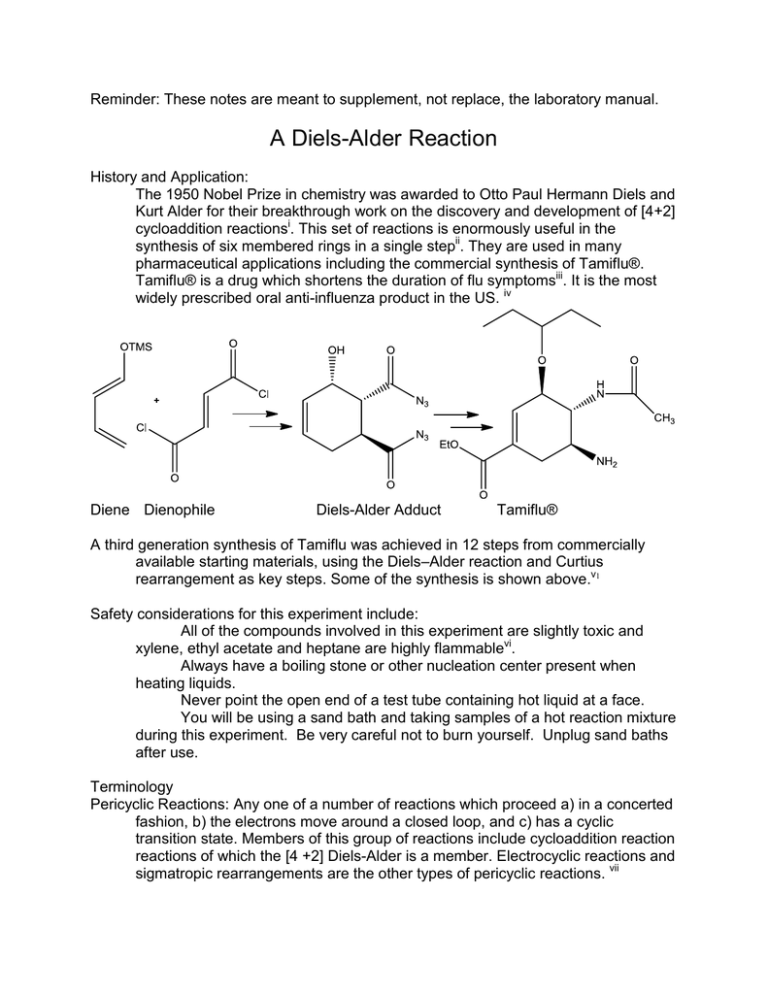
Reminder: These notes are meant to supplement, not replace, the laboratory manual. A Diels-Alder Reaction History and Application: The 1950 Nobel Prize in chemistry was awarded to Otto Paul Hermann Diels and Kurt Alder for their breakthrough work on the discovery and development of [4+2] cycloaddition reactionsi. This set of reactions is enormously useful in the synthesis of six membered rings in a single stepii. They are used in many pharmaceutical applications including the commercial synthesis of Tamiflu®. Tamiflu® is a drug which shortens the duration of flu symptomsiii. It is the most widely prescribed oral anti-influenza product in the US. iv Diene Dienophile Diels-Alder Adduct Tamiflu® A third generation synthesis of Tamiflu was achieved in 12 steps from commercially available starting materials, using the Diels–Alder reaction and Curtius rearrangement as key steps. Some of the synthesis is shown above.v1 Safety considerations for this experiment include: All of the compounds involved in this experiment are slightly toxic and xylene, ethyl acetate and heptane are highly flammablevi. Always have a boiling stone or other nucleation center present when heating liquids. Never point the open end of a test tube containing hot liquid at a face. You will be using a sand bath and taking samples of a hot reaction mixture during this experiment. Be very careful not to burn yourself. Unplug sand baths after use. Terminology Pericyclic Reactions: Any one of a number of reactions which proceed a) in a concerted fashion, b) the electrons move around a closed loop, and c) has a cyclic transition state. Members of this group of reactions include cycloaddition reaction reactions of which the [4 +2] Diels-Alder is a member. Electrocyclic reactions and sigmatropic rearrangements are the other types of pericyclic reactions. vii Cycloaddition Reaction- Two molecules come together and form one new cyclic compound. The HOMO of one molecule interacts with the LUMO of the other molecule. The [4+2] Diels-Alder is the most common of the cycloaddition reactions. Other cycloaddition reactions include the thermally allowed [6+4] and the photochemically allowed, but thermally prohibited [2+2]. Concerted Reactions- These are chemical reactions which occur in a single step. There is no intermediate between starting material and product. Bond breaking and bond formation occur simultaneously. Refluxing- A state of a liquid boiling and the vapors condensing and returning to the vessel. During reflux, the temperature, volume, and composition will remain fairly constant. Diene- A molecule containing two pi bonds. Diels-Alder reactions only work if the pi bonds are conjugated and can obtain a s-cis orientation. Dienes undergoing Diels-Alder reactions often have an electron donating group attached. Dienophile- A pi bond with reacts with the diene in a Diels-Alder reaction. Dienophiles undergoing Diels-Alder reactions normally have an electron withdrawing group attached. Adduct- This is a product which is formed by the addition of two or more groups or compounds. viii 1. The Diels-Alder reaction is a member of a class of reactions called cycloadditions. In all Diels-Alder reactions, three π bonds, two in a diene and one in a dienophile, reorganize to give a six-membered ring containing one π bond and two new sigma bonds. All of the bonds move at once, in a concerted manner, not stepwise. Other examples of concerted reactions include SN2 and E2 reactions. In contrast, SN1 and E1 reactions are not concerted, because they involve multiple steps and the formation of an intermediate (a carbocation). The illustration shows the movement of bonds. The structure in the center is the transition state and not an intermediate. ix 2. Dienes may be conjugated, cumulated or isolated. The diene which participates in a Diels-Alder reaction must be conjugated. 3. Many dienes can assume more than one conformation; that is, they have forms that can change from one to the other by rotation around single bonds. In 1,3butadiene (shown here), the s-cis conformation changes to the s-trans conformation by rotation of the single bond between the π bonds. (Note: Dienes react to form Diels-Alder adducts only in the s-cis conformation.) Most, but not all, dienes freely rotate between their s-cis and s-trans conformations. Note, neither of the individual pi bonds in butadiene are cis nor trans because they both only have one substituent. Conformations of 1,3 butadiene: S-trans S-cis 4. Anhydrides are compounds that have two acyl groups attached to an oxygen. They can be thought of as two acids that have joined together with the removal of a water molecule from the center. Anhydrides are relatives of ethers and esters, as shown here. Anhydrides can be cyclic or not, as illustrated by the examples below. First a generic example is shown, directly below is a specific example. ether carboxylic acid ester anhydride diethyl ether acetic acid ethyl acetate acetic anhydride maleic acid maleic anhydride 5.In today’s experiment anthracene is reacted with maleic anhydride. It looks a bit complicated because the central ring of the three membered aromatic compound is the one that will react as the diene. The diene is outlined in red. (Do not include the red highlight when writing this reaction in your lab notebook.) anthracene maleic anhydride 9,10-dihydroanthracene-9, 10-succinic acid anhydride or simply product 6. Here are the structures besides the reactants involved in this experiment. Xylene is the reaction solvent, a mixture of heptane and ethyl acetate (80:20) is used as the TLC solvent. 7. As the number of conjugated bonds increases, the wavelength of maximum electromagnetic radiation absorption (max increases. If a sufficient number of bonds are present the max will shift out of the UV range and into the visible range. The compound will then appear colored. If a highly conjugated system decreases the number of conjugated bonds the max will shift toward shorter wavelength and a colored compound may become clear. The more conjugated bonds, the longer wavelength max will be. (Re-read Klein 17.11&17.12) Shorter …. x-rays- UV-Visible (VIBGYOR)-IR-radio….. Longer 8. The progress of the reaction will be monitored using TLC. Reread the on-line notes from 2230 associated with this technique. The mobile phase will be an 80:20 heptane: ethyl acetate blend. You will have to make a TLC chamber. A 100 mL beaker is a perfect size. The atmosphere of the TLC chamber should be saturated with vapor to minimize evaporation from the TLC plate. A piece of filter paper placed within the TLC chamber with an edge submerged in the solvent works well for this. Keep the chamber tightly covered when not taking plates in or out. 9. For this experiment you will spot your plates with both starting materials, the solvent, and the reaction mixture. None of the materials are visible on the white TLC plates under visible light. Place them under UV light to see. Anthracene produces an intense spot under UV light, so just one tiny spot is sufficient for this material. The product is not very soluble in the solvent, so spot that sample in the exact same location multiple times. Each material applied on the bottom of the TLC plate makes its own lane, akin to a lane of traffic. Below on the left is a TLC plate labeled “start”. This is similar to what each of your TLC plates should look like before they are placed in the TLC chamber. The plates to the right indicate possible results after development. The number of spots in the reaction mixture lane, indicates the number of compounds contained in the reaction mixture. If only one spot is present in a lane, then that sample contains just one material and is pure. The Rf of each reaction mixture spot is compared with the Rf of the authentic starting materials to identify the spot. If a reaction mixture spot has an Rf different than both starting materials (SM), then that indicates the composition is not either SM and indicates a new product. Think about what defines when a reaction is complete. How will this translate to a TLC result? A reaction is complete when no more limiting reagent is present in the reaction mixture (because it is used up and hence no more product can form.) Identify the status of the reaction in I-IV above. 10. “Xylenes” means a mixture of ortho, meta and para xylene. Each of these materials has a slightly different boiling pointx. b.p. = 144.4oC b.p.= 139.1C b.p.= 138.3oC A mixture of these three xylenes will have a boiling point somewhere between 135-145 depending upon the exact composition of the mixture. Notes for topics that may be included in quizzes given after the experiment: 11. Some features of this experiment include: If you fail to dry the product completely, it will include some solvent and you may obtain a percent yield that is unrealistically high, possibly over 100%. If the reaction was not allowed to proceed for a sufficient amount of time, or at too low a temperature, the reaction will not have gone to completion. The reaction will not have finished. There will be both starting materials and the desired product in the reaction mixture. This means the reaction did not continue until all of the limiting reagent was consumed. To speed the reaction, one cannot simply raise the variac to 40%, because the temperature of the reaction mixture is the temperature of the boiling solvent. Since the xylene has a constant boiling temperature raising the setting will increase the rate of boiling but will not increase the temperature of the reaction. Revised: S.L. Weaver, February 11, 2016 i : "The Nobel Prize in Chemistry 1950". Nobelprize.org. 8 Feb 2011 http://nobelprize.org/nobel_prizes/chemistry/laureates/1950/ ii Fringuelli, F., Taticchi, A., The Diels-Alder Reaction. Selected Practical Methods, Wiley, New York 2002 pp 1-25. “Prescribing Tamiflu ” http://www.tamiflu.com/hcp/prescribing/hcp_prescribe.jsp (accessed February 7, 2011) iv “ xxx” http://www.fda.gov/ohrms/DOCKETS/ac/05/briefing/20054180b_06_02_Tamiflu%20Drug%20Use%20Review%20Cleared.pdf (February 7, 2011) v1 Kenzo Yamatsugua, Shin Kamijoa, Yutaka Sutoa, Motomu Kanai , a and Masakatsu Shibasaki , a, Tetrahedron Letters Volume 48, Issue 8, 19 February 2007, Pages 1403-1406 vi Sigma-Aldrich SDS Xylenes, http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=2 47642&brand=SIAL&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Fsia l%2F247642%3Flang%3Den (accessed February 11, 2016) vii Klein, D, Organic Chemistry, Wiley, 2012, p783. viii The Oxford English Dictionary, On-line, 3rd Edition, November 2010, ix Organic Chemistry, David Klein, Wiley, Hoboken NJ, 2012, p784 x CRC Handbook of Pure and Applied Chemistry, CRC Press, 1985, C-573 iii