8. Infrared Spectroscopy
advertisement

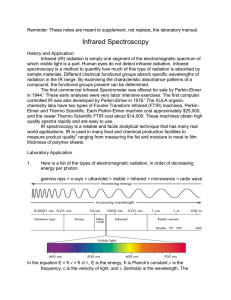
8. Infrared Spectroscopy Infrared (IR) Spectroscopy is an analytical method that measures the absorbance of a select band of electromagnetic radiation by a sample. The wavelength of radiation that is absorbed is characteristic of different types of chemical bonds. The graphical representation of the absorption of the electromagnetic radiation by the sample makes an IR spectrum. Organic chemists use infrared spectroscopy as a means of identifying various types of bonds within in a molecule which determine the functional groups present or absent. This information is used to determine the structure of compounds. IR is usually used in combination with other techniques, especially nuclear magnetic resonance (NMR) spectroscopy. In this class you will learn how to interpret an IR spectrum and how to operate a Perkin-Elmer and/or Nicolet infrared spectrometer. During this experiment you will obtain an IR spectrum of an unknown compound and use this information to identify the functional groups present and select the identification of the material from a list of possible materials. PRE-EXPERIMENT ASSIGNMENT Study this chapter of the manual, the lecture notes on the Chemistry Department web site, the “Sample IR Spectra” posted on the web site and the appropriate section in your lecture textbook. Complete the take home assignment relating to Infrared. This will be due at the very beginning of the lab period. A major part of this lab will be in operating an Infrared Spectrometer. Your instructor will explain and show you how to do this. Since all the steps involve operation of equipment, a pre-lab notebook procedure does not need to be written. It will however be very important for you to write down in your notebook all of the possible unknown compounds from the on-line notes. If you do not have this information, it will be very difficult for you to successfully determine the identity of your unknown material and points will be lost. The notebook however will not be graded. In lieu of these points, an in-class problem set will be assigned. A student who has prepared for the Infrared Spectroscopy experiment should be able to: 1. Identify the relative energy, frequency, and wavelength of infrared light compared with other types (including gamma rays, x rays, ultraviolet and visible light, microwaves, and radio waves). Also, identify the change that occurs in molecules when infrared light is absorbed. 2. Explain the relationship between frequency, energy, wavelength and wavenumbers. 3. Define the major absorbance regions in the IR. Know the frequency which each major functional group absorbs in the IR. 4. If given a functional group, choose in which of the 5 regions it will absorb. 5. Define, identify, recognize, and explain the use of each of the following: IR (infrared) spectroscopy, neat samples, salt plates (including how to clean them), Nujol mull, and KBr pellet. 6. Draw the structure given the name, or give the name from the structure, of the compounds used in the day's experiment. 7. Identify and explain safety considerations for this experiment. 8. Perform the day's experiment safely and successfully. Quizzes given after the experiment has been performed may also include: 9. Give or recognize a probable absorption frequency in cm-1 in the IR spectrum of a compound containing any of the following functional groups; Amines, Alcohols, Alkynes, Terminal Alkynes, Nitriles, Alkanes, Alkenes, Aldehydes, Carboxylic Acids, Esters, Amides, Aldehydes, Ketones, and Ethers. (Detailed instructions of spectrum interpretation can be found in “Sample IR Spectra” located on the organic web site. 10. Draw a reasonable IR spectrum if given a compound name or structure. 11. Determine the presence (or absence) of functional groups in a sample from infrared spectral data. The data may be given in the form of a list of peak locations (see #6 above) or as a spectrum. Safety Precautions All of the compounds you will take IR spectra of in this experiment are at least slightly toxic and flammable. Wash your hands after conducting the experiment. Infrared radiation is relatively (but not completely) harmless. The safety instructions on the IR spectrometer say “Do not stare into beam,” because long exposures to IR radiation can be damaging. EXPERIMENT Preparing the sample Handle salt plates with care. The salt plates are very sensitive to moisture and will break easily. Each pair costs approximately $25. If you are the first student, remove the jar containing the salt plates from the desiccator. (A desiccator is a low humidity vessel.) Remove two salt plates from cotton envelope. Place the two salt plates on a Kim-Wipe. Clean plates by placing a few drops of acetone on each salt plate, then wiping clean with Kim-Wipe. Throw Kim-Wipe away. Place 1-2 drops of unknown liquid on one salt plate. Cover with other salt plate. Carefully place in sample holder. Take sample to IR to obtain spectrum. Obtaining the IR spectrum Take sample to IR spectrometer. Follow instructions posted adjacent to machine. Cleaning the salt plates Remove salt plates from sample holder. Place salt plates on Kim-Wipe. Place a few drops of acetone on each salt plate. Using a new Kim-Wipe, rub each salt plate with the acetone. If another student is waiting to run the spectrometer, help them go through the process. If you are the last user, replace each salt plate in a separate cotton envelope. Place each envelope within the plastic jar. Place the plastic container back in the desiccator. Throw away any used Kim-Wipes. Cap your unknown sample. Replace unknown sample in its designated spot within the unknown sample container. POST-EXPERIMENT ASSIGNMENT Complete the datasheet and turn in before leaving class. Ensure your IR spectrum is stapled to the datasheet. Turn in the white notebook pages from your lab notebook. Staple multiple sheets together. Tear off rough edges. Prepare for the IR portion of the next quiz. Remember that you may be asked questions on anything you should have learned from assigned readings, pre-experiment lecture, or doing the experiment. Revised March 27, 2016 S. L. Weaver Functional Groups Position (cm-1) O-H/ NH 2500-3500 (Nitrogen & Oxygen to Hydrogen) -O-H 3200-3500 -N-H 3200-3500 -COOH 2500-3500 C-H (Carbon to Hydrogen) sp ≡C-H sp2 =C-H sp3 C-H 2800-3100, 3300 ≡ (Triple Bond) C≡N C≡C-H 2100-2300 = (Double Bond) C=O C=C 1620-1800 Fingerprint -C-O- <1600 1000-1300 3300 3000-3100 2800-3000 2200-2300 2100-2260 1650-1800 1620-1680 Shape strong, rounded medium, pointed very broad strong, very pointed meduim, shoulder multi-peaked weak sharp very weak sharp strong medium, sharp limited value use only when necessary Complete Table of Main IR Frequencies Wave number, cm-1 3200-3400 Functional Group -O-H (alcohol) Peak Description Strong and broad 2500-3500 -O-H (carboxylic acid) Very broad (over ~ 500 cm-1), often looks like distorted baseline, can be above 3000-1. 3200-3500 N-H Doublet in case of NH2 group of a primary amine or amide. 3300 ≡C-H ( sp carbon-H, terminal alkyne) Usually sharp and strong 3000-3100 =C-H (alkene or arene) Often weak, overlaps with C-H alkane absorption. Looks like shoulder on other peak. 2800-3000 C-H (sp3 carbon) Strong, broad and multi-banded 2200-2500 C≡N Medium intensity 2100-2260 C≡C (alkyne) Medium intensity for terminal alkynes, very weak for internal alkynes. 1650-1800 C=O (carbonyl of amides, ketones, aldehydes, carboxylic acids and esters) Very strong; lower frequency for amides and when C=O is conjugated. 1620-1680 C=C (alkene, aromatic ring) Check to see if you have a sp C-H unsaturated stretch at >3000cm-1 (if not, it is completely substituted) ~1600 -NH2 (bending 1o amines and amides) Only if you have corresponding N-H peak at 3200-3500 cm-1 (otherwise this peak may be mistaken for C=C). 1200 Ar-H 1000-1300 690 and 750 C-O Phenyl group Strong (look for =C-H and C=C first, this area easy to misidentify) Easy to misidentify. Strong (look for =C-H and C=C first, this area easy to misidentify)