Good Lab Skill Reminders General
advertisement

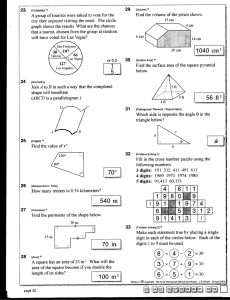
Good Lab Skill Reminders General 1. Be sure you know what you are doing in lab before ever stepping foot inside the door. 2. Always wear safety classes and closed shoes when anyone is working inside the laboratory. Do not take off goggles until ALL chemicals are put away in entire lab. 3. If you are unsure of how to do a procedure safely, STOP. Ask for directions or clarification. 4. Always check the label of reagent bottles or the waste container you plan to use. 5. Always clean up after yourself. If your neighbor has already departed and left some things undone, go ahead and clean up after them. Tell them the next time you see them. Data Related 1. Always write your data directly into your lab notebook. Do not first write your data on another paper and transfer into notebook later. Take your notebook with you to the balance. 2. Write in your notebook using pen not pencil. Do not erase. 3. Always write all the digits the balance provides. If the balance display is “2.000 g” then write this down. This is a very different number than 2 g. 4. If the procedure asks for about 0.08 grams, do not waste time and effort getting EXACTLY 0.0800 grams of the material on the balance. Instead, weigh out an amount close to 0.0800 g and then record all the digits, such as 0.0758 g or 0.0843 g. 5. When manipulating your data, always use all of the digits you obtained. Do not arbitrarily drop off digits. (Do drop digits in answer according to significant figure rules.) 6. Do not arbitrarily add zeros to the end of a number. This is enhancing the precision of a measurement. Fabricating and altering data is a serious offence. Do not do this. 7. Remember to record your observations into your lab notebook and not just your hard data. Often what you see is just as important as what you measure. 8. When reading a scale such as a graduated cylinder, burette, or ruler, record to one digit past the smallest mark. (A good practice program can be found at the following https://www.youtube.com/watch?v=7ewRaV5baik ). This pencil is 4.03cm in length. This volume is 42.5 mL The temperature is 86.4o C The burette volume is 24.28mL This reading is 1.3347 Significant Figures 1. Some digits are ‘exact’ numbers and have an infinite number of significant digits. For example, there are 12 eggs in a dozen. Even though number 12 only has two digits, it possesses an infinite number of significant digits because its value is known exactly. 2. Leading zeros are never significant. These are the zeros to the left of the first non-zero numeral. e.g. 0.00123 has 3 significant digits. (It helps to see when put into scientific notation, 1.23 x 10-3.) 001,230,000 has 3 significant digits 1.23 x 106 3. Trailing zeros are significant only if the decimal point has been specified or other information is known. These are the zeros to the right of the non-zero numbers. e.g. 123,000 has 3 significant digits. 123,000.0 has 7 significant digits 20 has 1 significant digit 20. has 2 significant digits. This also can be written 2.0 x 10 1 2 has 1 significant digit 2.00 has 3 significant digits 2.000 has 4 significant digits. The zeros to the right are important. 4. Middle zeros are always significant. These are zeros between non zero numbers. e.g. 1098 has 4 significant digits 5. When multiplying or dividing, determine the number of significant digits in each numeral which will take part in the operation. The answer will have the same number of significant digits as the least significant number. 3.709 x9.827 x0.0072 0.029159 Error; 0.02916 Error; 0.0292 Error, 0.03 Error, 0.0029 CORRECT 9.00 The first two numerators have 4 sig. figs. The last numerator has 2 s.f. The denominator has 3 sig figs, hence the answer must have 2 significant digits. 6. When adding or subtracting, drop the digits on the right of the decimal point that each number does not posses. (You may add, and then round to the correct number of places) 4.2236 0.0428 5.710012 0.0218 2.3 0.021 ERROR + 1.907 0.0210 CORRECT 14.140612 ERROR 14.1 CORRECT Whichever column is not completely filled, is NOT significant ??Question. When averaging the following numbers 923.995, 131, 127.85, how many digits will the correct answer have? This is not obvious. [The sum of the numbers has no decimal places due to addition rules. This number has 4 significant figures. This is then divided by the exact number 3 with an infinite number of significant figures, so the answer also has 4 significant figures, 394.0] Here is a very useful link to other significant figure help. http://www.chem.tamu.edu/class/fyp/mathrev/mrsigfg.html (Revised February 19, 2016 slw)