Aldehydes and Ketones: Organic Chemistry Study Guide
advertisement

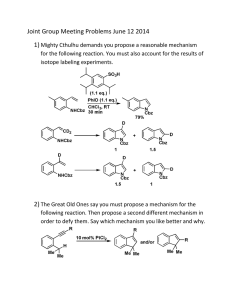
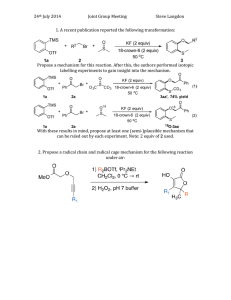
ALDEHYDES AND KETONES IN WEEK 1, A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name given the structure, and draw the structure given the name, of aldehydes and ketones. Also, draw the structure given any of the following common names: formaldehyde, acetaldehyde, benzaldehyde, acetone, acetophenone. 2. Predict the products of reactions giving rise to aldehydes and ketones, given the starting material and reaction conditions. Important reactions include: Oxidation of 1° and 2° alcohols Ozonolysis of alkenes Hydroboration of alkynes Acid-catalyzed hydration of alkynes 3. Predict the products of the reactions of aldehydes and ketones. Important reactions include: Reactions with hydrides (reducing agents such as LiAlH4 and NaBH4) Reactions with water and alcohols (to form hemiacetals, acetals) Reactions with derivatives of ammonia (to form oximes, semicarbazides, hydrazones, imines, and enamines) Reactions with thiols Oxidation Wolff-Kishner reduction of ketones to alkanes 4. Propose syntheses using the reactions of Objectives #2 and #3. 5. Propose mechanisms of reactions involving nucleophilic attack on the carbonyl carbon. 6. Predict and interpret spectra. IN WEEK 2, A STUDENT SHOULD ALSO BE ABLE TO: 7. Perform the objectives detailed above for these additional reactions with an emphasis on C-C bond forming reactions in synthesis: Friedel-Crafts acylation Reactions with Grignard and organolithium reagents Reactions with ylides (Plus other reactions in the Wittig reaction sequence) To best prepare for this module, please work Chapter 20 Skill Builder problems in the textbook. A STUDENT WHO HAS MASTERED THE OBJECTIVES ON THE PREVIOUS PAGE SHOULD BE ABLE TO SOLVE THE FOLLOWING PROBLEMS AND RELATED ONES: Problems 1-6 should be mastered for the first week of drill: 1.1 Draw each of the following compounds. a) acetaldehyde b) acetophenone c) 2-methylcyclopentanone 1.2 Name each of the compounds shown. 2. Predict the product(s) of each of the following reactions. a) d) 1. O3 2. Me2S 2. 3. Predict the product(s) of each of the following reactions. 4. Propose syntheses of each of the compounds indicated, from the given starting material and any other needed reagents. a) 3-methyl-1-butanol from trans-5-methyl-2-hexene 5. Propose a mechanism for the following reactions. 6.1 Propose structures for each of the following compounds: (a) C9H10O2 IR: 1695 cm-1 1 H NMR spectrum triplet, 1.3 (3H) quartet, 4.1 (2H) doublet, 6.9 (2H) doublet, 7.8 (2H) singlet, 9.9 (1H) 6.1 continued (b) C10H12O IR: 1710 cm-1 1 H NMR spectrum triplet, 1.0 (3H) quartet, 2.2 (2H) singlet, 3.8 (2H) singlet, 7.2 (5H) 7.1 Predict the product(s) of each of these reactions. 7.2 Complete these reactions. 7.2 continued OH e) 7.3 1. CH3CH2Li 1. H2CrO4 2. H3O+ 2. CH3OH, H+ Propose syntheses of each of the compounds indicated. a) benzaldehyde from benzene 7.3 continued 7.4 Propose a mechanism for this reaction: SOLUTIONS TO SAMPLE PROBLEMS: 1.1 a) acetaldehyde b) acetophenone 1.2 a) 4-methyl-2-pentanone b) 3,3-dimethylcycloheptanone c) 3-chloropropanal c) 2-methylcyclopentanone 2. The reactions give these products: a) b) 2. H2O2, NaOH 3. O 1) BH3-THF H 4. 5. Mechanisms: a) - OCH3 O + HOCH3 CH3O + CH3O- HO basic solution: neutrals and anions, not cations OCH3 O- H CH3 O 6.1 7.1 Unknowns: 7.1 continued 7.2 O O a) + Cl AlCl3 7.3 d) (C6H5)3P + CH3CH2Br → (C6H5)3P+CH2CH3 Br(C6H5)3P+CH2CH3 Br- + NaNH2 → (C6H5)3P=CHCH3 (C6H5)3P=CHCH3 + C6H5CH2CH=O → C6H5CH2CH=CHCH3 + O=PPh3 7.4 O C OMgBr OCH2CH3 + 2 CH3MgBr C(CH3)2 + CH CH OMgBr 3 2 O C OMgBr OCH2CH3 + CH3MgBr C OCH2CH3 CH3 OMgBr O C C OCH2CH3 CH3 + CH3CH2OMgBr CH3 O C OMgBr CH3 + CH3MgBr C(CH3)2 Name __________________________________________________ Drill Test 6.1 (Sample A) Organic Chemistry 2220D Answer All Questions 1. Draw the structure of: p-nitroacetophenone 2. Give the IUPAC name of: 3. Predict the products of these reactions: a. b. c. d. e. 4. Propose complete mechanisms for these reactions. Include all intermediates and arrows. a. b. 5. Propose syntheses for these compounds: a. 2-butanol from 3-methyl-3-heptene b. 6. Draw the structure for a compound that is consistent with these data: IR: 2950 cm-1; 1750 cm-1 13 C NMR: 212, 42, 27, 18 ppm 1 H NMR: 2.6 ppm, septet, 1H; 2.1 ppm, singlet, 3H; 1.1 ppm, singlet, 1H m/z of molecular ion: 86 Name __________________________________________________ Drill Test 6.1 (Sample B) Organic Chemistry 2220D Answer All Questions 1. What is the IUPAC name of: 3. Predict the products of these reactions: 2. Draw the structure of: 2,2,2-trichloroacetaldehyde 3. 4. Propose syntheses. a. benzaldehyde from benzyl chloride b. c. CH3CH2CH2CH=NH from CH3CH2C≡CH 5. Draw a mechanism for each of these reactions: 6. Predict the IR and 1H NMR spectra of 3-pentanone. Name ________________________________________________ Drill Test 6.2 (Sample A) Organic Chemistry 2220D Answer All Questions 1. Name: O Cl Cl 2. Draw the structure of (a) 1,1-diiodo-3-pentanone 3. Predict the product or products (if any) of each of the following reactions. dil H+ d) CH3CH2C≡CH + HgSO4, H2SO4, H2O → (b) 2-hydroxypropanal 4. Propose a synthesis of each of the compounds shown, from the given starting material and any other needed reagents. S S a) from b) f rom (C6H5)3P and CH3Cl Br c) f rom O Name ________________________________________________ Drill Test 6.2 (Sample B) Organic Chemistry 2220D Answer All Questions 1. Which of the following compounds gives a new product on reaction with H2CrO4, and decolorizes Br2 in CCl4? 2. Give the IUPAC name for the following compound. 4. Predict the product of each of the following reactions. 5. Propose a synthesis of the compounds indicated from the given starting materials and any other needed reagents. 3. Draw the structure of 2,3-dimethylcyclopentanone. 6. 7. Propose a mechanism for the reaction shown. An unknown has the formula C4H6O and a strong peak in the IR spectrum near 1690 cm-1. The pmr spectrum is: doublet, 2.0, 3H multiplet, 6.1, 1H multiplet, 6.9, 1H doublet, 9.5, 1H Give the structure of this unknown.