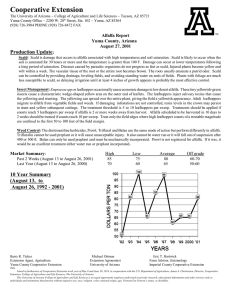
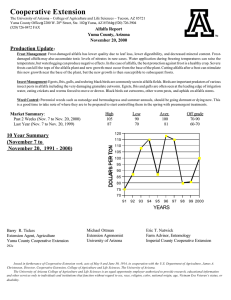
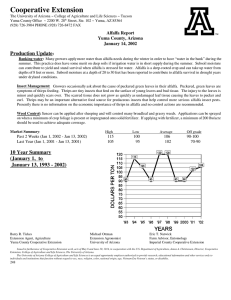
AN ABSTRACT OF THE THESIS OF
advertisement

AN ABSTRACT OF THE THESIS OF Philip E. Shuler for the degree of Doctor of Philosophy in Crop Science presented on July 19, 1991. Title: The Effect of Preplant Nitrogen Fertilization and Soil Temperature on Biological Nitrogen Fixation and Yield of Alfalfa (Medicago sativa La._ Abstract approved: Redacted for Privacy David 5. Hannaway The usefulness of preplant nitrogen (N) in establishing alfalfa in colder production areas has not been well characterized. This study was conducted to deter- mine the effect of preplant N and soil temperature on yield, percent N derived from biological nitrogen fixation (PBNF), and shoot N concentration in alfalfa (Medicago sativa L. cv. 'Vernema'. Field experiments were conducted in 1987 and 1988 at Powell Butte, OR, to determine the effect of five levels of preplant N (0, 10, 20, 40, 60 kg ha-1) on yield and shoot N concentration of alfalfa. Growth chamber experiments were conducted from 1989 through 1991 to examine the effect of five levels of preplant N (0, 10, 20, 40, 80 kg ha-1) and three day/ - night soil temperatures (18/12°C, 24/16°C, 27/21°C) on yield, PBNF, and shoot N concentration of alfalfa. In field experiments, preplant N had no effect on shoot N concentration in either year. In 1987 there was no effect of preplant N on dry matter yield. Application of 20-40 kg N ha-1 preplant N increased dry matter yield in 1988. In growth chamber experiments, the highest rate of dry matter accumulation occurred at a soil temperature of 24/16°C. At 18/12°C and 24/16°C, 40 kg ha-1 preplant N resulted in increased shoot and root dry matter yield. At 18/12°C, 80 kg ha-1 preplant N increased PBNF 14% relative to the zero N control. There was no effect of preplant N on PBNF in plants grown at 24/16°C and 27/The rates of shoot N accumulation were similar at 21°C. 18/12°C and 24/16°C, and were higher than at 27/21°C. Shoot N concentration was not affected by preplant N treatments. The use of 20-40 kg ha-1 preplant N may result in increased yield without decreasing PBNF when: 1) soil temperature remains below 15°C for at least two weeks after planting, and 2) soil nitrate level is less than 16 mg kg-1. Proper assessment of the use of preplant N in alfalfa establishment requires a careful consideration of both soil temperature and soil N availability. The Effect of Preplant Nitrogen Fertilization and Soil Temperature on Biological Nitrogen Fixation and Yield of Alfalfa (Medicago sativa L.) by Philip E. Shuler A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Completed July 19, 1991 Commencement June 1992 APPROVED: 'Redacted for Privacy Associate Professor of Crop Science charge of major _Redacted for Privacy Head of Crop and Soil Science Department Redacted Dean of Privacy _ Date thesis is presented July 19, 1991 Typed by Philip E. Shuler for Philip E. Shuler ACKNOWLEDGEMENTS The process of earning a doctoral degree in science is truly an exhaustive one. To state that the demands placed on the individual are severe is an understatement. These stringent demands also have a significant impact on family, friends, and professional colleagues over a In this sense the degree program is not number of years. in any way a strictly individual effort. Therefore, at the completion of such a rigorous journey, an expression of genuine appreciation to my colleagues and friends is very much in order. I extend my sincere gratitude to the head of the Crop and Soil Science Department, Dr. Sheldon Ladd and my major professor, Dr. David Hannaway, for financial support in the form of an assistantship. Dr. Ladd's concern and willingness to help demonstrated a richness of character that should be imitated by every Appreciation is also extended to the members of my graduate committee: Dr. Dale Moss, Dr. Tim administrator. Righetti, Dr. Tom Grigsby, and Dr. Barry Schrumpf, all of whom have had a unique and positive contribution to my degree program. A special thanks also goes to the friendly, ever helpful secretarial staff including Doris Aponte, Sue Ferdig, Irene Lampert, and Jenelle Post. Their sharing of clerical skills and personal encouragement was an essential part of my educational process. Friends and family are a priceless treasure and have provided much essential support during this arduous quest. They have given me the freedom to explore a number of "roads less traveled" with patience and encouragement. The St. John family has been a constant source of support throughout my education process. Their faithful encouragement and support for me through many years have resulted in improved standards of scholarship and conduct. To my parents, Woodfin and Ethel Shuler, I They have owe an enormous debt of respect and honor. helped me develop a drive for continuing education, a concern for integrity in scholarship, and an ability to persevere through the tough times in life. They have been a model of hard work and service to all of their children. The choice fruit of their labors will last us a lifetime. Finally, a humble, heartfelt thanks must surely go to David and Kimberly Hannaway. In them I have seen a timeless and beautiful principle brought to life: "Do not forget to do good and to share, for with such sacrifices God is well pleased". TABLE OF CONTENTS Page I. II. INTRODUCTION 1 Objectives 4 REVIEW OF LITERATURE Introduction 5 BNF in Alfalfa 6 The Effect of Combined Nitrogen on BNF in Alfalfa III. 5 10 The Use of Preplant N in Alfalfa Production 14 The Effect of Temperature on Growth of Alfalfa 19 The Effect of Temperature on BNF in Alfalfa 21 Methods of Measuring Biological Nitrogen Fixation 23 The Difference Method 25 THE EFFECT OF PREPLANT NITROGEN FERTILIZATION AND SOIL TEMPERATURE ON BIOLOGICAL NITROGEN FIXATION AND YIELD OF ALFALFA (MEDICAGO SATIVA L.) 28 Abstract 28 Introduction 30 Materials and Methods 32 Field Experiments 32 Growth Chamber Experiments 35 Statistical Analysis 38 Results and Discussion 39 Field Experiments 39 Growth Chamber Experiments 45 Dry Matter Yield 45 PBNF and N Accumulation 52 TABLE OF CONTENTS (Cont.) Page IV. V. Conclusions 58 References 62 BIBLIOGRAPHY 66 APPENDIX 76 LIST OF TABLES Page Table II1.1 111.2 111.3 111.4 111.5 111.6 111.7 111.8 111.9 111.10 Soil characteristics of field site at Powell Butte, OR and washed river sand used in growth chamber experiments before treatments applied. 40 Shoot N concentration of alfalfa grown at five levels of preplant N at Powell Butte, OR in 1987 and 1988. 41 Dry matter yield of alfalfa grown at five levels of preplant N at Powell Butte, OR in 1987 and 1988. 42 Combined analysis of variance of three day/night soil temperature regimes and five levels of preplant N in growth chamber experiments. 46 Shoot dry matter yield at harvest of alfalfa grown in three soil temperatures and five levels of preplant N in growth chamber experiments. 47 Linear regression analysis of shoot dry matter accumulation (SHDW) in response to thermal time (GDIDO and preplant N in growth chamber experiments. 48 Root dry matter yield at harvest of alfalfa grown in three soil temperatures and five levels of preplant N in growth chamber experiments. 50 Percent N derived from biological nitrogen fixation (PBNF) in alfalfa grown in three soil temperatures and five levels of preplant N in growth chamber experiments. 53 Linear regression analysis of shoot N accumulation (SHN) in reponse to thermal time (GDDO and preplant N in growth chamber experiments. 54 Shoot N concentration at harvest of alfalfa grown in three soil temperatures and five levels of preplant N in growth chamber experiments. 55 LIST OF APPENDIX TABLES Table 1. 2. 3. 4. 5. 6. 7. 8. Page Analysis of variance of dry matter yield for 1987 and 1988 field experiments at Powell Butte, OR. 76 Shoot and root dry matter accumulation of alfalfa grown at a day/night soil temperature regime of 18/12°C and five levels of preplant N in growth chamber experiments. 77 Shoot and root dry matter accumulation of alfalfa grown at a day/night soil temperature regime of 24/16°C and five levels of preplant N in growth chamber experiments. 78 Shoot and root dry matter accumulation of alfalfa grown at a day/night soil temperature regime of 27/21°C and five levels of preplant N in growth chamber experiments. 79 Shoot and root N concentration of alfalfa grown at a day/night soil temperature regime of 18/12°C and five levels of preplant N in growth chamber experiments. 80 Shoot and root N concentration of alfalfa grown at a day/night soil temperature regime of 24/16°C and five levels of preplant N in growth chamber experiments. 81 Shoot and root N concentration of alfalfa grown at a day/night soil temperature regime of 27/21°C and five levels of preplant N in growth chamber experiments. 82 Shoot and root N content of alfalfa grown at a day/night soil temperature regime of 18/12°C and five levels of preplant N in growth chamber experiments. 83 LIST OF APPENDIX TABLES (Cont.) Table 9. 10. Page Shoot and root N content of alfalfa grown at a day/night soil temperature regime of 24/16°C and five levels of preplant N in growth chamber experiments. 84 Shoot and root N content of alfalfa grown at a day/night soil temperature regime of 27/21°C and five levels of preplant N in growth chamber experiments. 85 THE EFFECT OF PREPLANT NITROGEN FERTILIZATION AND SOIL TEMPERATURE ON BIOLOGICAL NITROGEN FIXATION AND YIELD OF ALFALFA (MEDICAGO SATIVA L.) I. Introduction Alfalfa is one of the most important forage crops cultivated in the U.S. (Barnes et al., 1988). In Oregon, it is grown on about 400,000 acres with commercial sales of over $60,000,000 and a farm-gate value of about $150,000,000 (Oregon Department of Agriculture, 1990). Proper management of alfalfa can be a formidable task given the complexity of the interaction between alfalfa, its microsymbiont Rhizobium meliloti, and the environment. The use of fertilizer N is an important and controversial issue in alfalfa production. In a survey of 45 U.S. agronomy departments, Hojjati et al. (1978) reported that 17 recommended N in legume establishment, at rates of 22.4-67.3 kg ha-1. Some alfalfa producers have used pre- plant nitrogen fertilizer (N), also known as "starter N", in the establishment of alfalfa stands. Research results have varied on the effects of preplant N on yield and biological nitrogen fixation in alfalfa. Some results show that starter N does not increase yield in alfalfa (Eardly et al., 1988; Simson et al., 1981) and that it reduces stands by increasing weed growth 2 (Peters and Stritzke, 1970). In field studies, Eardly et al. (1988) reported that the application of up to 90 kg ha - 1 preplant N did not result in significant dry matter yield increase in the first harvest of the seeding year. The studies showing no effect of preplant N were conducted on soils with relatively high N supplying capacity. Other reports show starter N increases yield in alfalfa (Peters and Kelling, 1989; Kunelius, 1974; Ward and Blaser, 1961). Two factors common to the observation of a positive response are low soil N and adequate weed control. The response in low N soils is explained by the assertion that alfalfa experiences a period of "N-starvation" in seedling growth. During this period, demand for N exceeds the ability of the root to supply N from soil N or BNF, particularly if BNF capacity has not developed (MacDuff et al., 1989). The application of mineral N to legumes affects BNF, from initial infection to subsequent growth and activity of nodules (Streeter, 1988). In general, the relationship between nodulation and mineral N, particularly nitrate, is an antagonistic one. Laboratory studies suggest that a nitrate concentration in growth media greater than 5 mM inhibits nodulation in legumes (Streeter, 1988). The nitrate concentration of the soil solution, which ranges 3 from 2-400 mg 1-1 in agricultural soils, is therefore often high enough to inhibit nodulation (Barber, 1984). Starter N, however, may at times stimulate development of BNF in alfalfa. Small amounts of combined N (below inhibitory levels) are required for maximum nodule growth and BNF in legumes (Mahon and Child, 1979; Allos and Bartholomew, 1959). Fishbeck and Phillips (1981) provide laboratory evidence for a period of N-deficient growth in alfalfa. During this period of "N- starvation ", BNF is not able to meet the N needs of the developing seedling. Thus, application of fertilizer N may lead to increases in BNF and yield in alfalfa in the establishment year. The conditions under which N applications will most likely result in increased yield without adversely affecting BNF are not well defined. Important factors in the response appear to be the N supplying ability of soils and soil temperature (Vance et al., 1988; Barta, 1978). More specific information on how these factors affect yield and BNF is needed to provide a scientific basis for making recommendations on using starter N in alfalfa establishment in Oregon. The purpose of this research project was to determine the effects of soil temperature and preplant N fertilization on yield, shoot N concentration, and BNF of 'Vernema' alfalfa in a low N soil. 4 Objectives Field and growth chamber studies were conducted to evaluate the effect of soil temperature and preplant N fertilization on yield, shoot N concentration, and BNF in alfalfa. The objective of the field experiments was to determine the effect of five levels of preplant N on shoot dry matter production and shoot N concentration of alfalfa in the establishment year. The objective of the growth chamber experiments was to determine the effect of five levels of preplant N and three soil temperature regimes on shoot dry matter accumulation, shoot N accumulation, and the percent N derived from BNF (PBNF). 5 II. REVIEW OF LITERATURE Introduction The relationship of plant growth to soil nitrogen (N) content is one of the most critical factors to be considered when dealing with the concerns of agricultural development into the next century (Winteringham, 1984). The relationship of soil N with alfalfa (Medicago sativa L.) production presents special challenges. These challenges arise primarily because of the ability of alfalfa to assimilate atmospheric N2 via the process of biological nitrogen fixation (BNF). This potentially lessens the need for fertilizer N applications both to alfalfa, and to crops following in a rotation. Significant progress has been made in understanding the physiology of BNF (Heichel et al., 1989). Less prog- ress has occurred in applying this knowledge to the practice of food production (Dreyfus et al., 1988). Critical questions remain regarding the contribution of alfalfa to the N economy of cropping systems through BNF. A major reason for this is the complexity of the interaction between alfalfa, its microsymbiont Rhizobium meliloti, and the environment. Soil N content, water availability, temperature, pest levels, and management play key roles in 6 determining seasonal levels of BNF. Knowledge of how these factors impact N accumulation and rates of BNF in alfalfa is incomplete. This information is needed to design management practices that will have a positive impact on N accumulation, BNF, and dry matter production. BNF in Alfalfa Alfalfa has a symbiotic association with Rhizobium meliloti, which provides a means to convert atmospheric N2 It fixes large amounts of atmospheric N2 to ammonia-N. and derives a large portion of it's N from BNF (Barnes et al., 1988a). This biologically assimilated or "fixed" N is incorporated into the alfalfa plant and contributes to the high yield and quality of alfalfa. The use of alfalfa in animal production and cropping systems as a replacement for fertilizer N has prompted much research into it's production and N2 fixing abilities. Research to maximize BNF in the legume/Rhizobium symbiosis has focused on three major areas: 1) infection by an effective strain of Rhizo- bium, 2) investment of N and other nutrients from the germinating seed to promote nodule formation, and 3) inhibition of nodulation and fixation by soil and fertilizer N (Atkins, 1986). Research to select cultivars with higher BNF has resulted in alfalfa populations with higher yield 7 and quality (Teuber et al., 1984). Studies of the morphology, physiology, and interac- tion between alfalfa and various Rhizobium strains show that BNF in alfalfa is affected by coordinated responses among many physiological and biochemical traits (Barnes et al., 1984). The symbiotic association in alfalfa begins with contact between roots and Rhizobium meliloti. This contact is assured when there are high native populations of effective rhizobia present. In soils that are acid or that have low populations of effective bacteria, seed inoculation is generally effective in insuring contact between roots and rhizobia (West and Wedin, 1985; Munns, 1977). Numerous studies have demonstrated that the strain of bacteria which colonizes the root significantly affects the rate of BNF (Barnes et al., 1984; Burton, 1972). Therefore, a careful matching of strain and cultivar is necessary to achieve maximum BNF (Burton and Erdman, 1940; Gibson, 1962). In field conditions this is often achieved by inoculating seed with a mixture of effective strains (Burton, 1972). Seasonal variation of BNF in field-grown alfalfa has been the subject of relatively few reports (Heichel et al. 1984; Henson and Heichel, 1984; Eardly et al., 1988; Wivstad et al., 1987). Estimates of BNF in alfalfa range from 50-463 kg N2 ha-1 yr-1, with an average of about 200 kg 8 N2 ha-1 yr-1 (Vance et al., 1988). Variation in these esti- mates is due to plant and environmental factors, including stand age (Heichel et al., 1984), genotype (Tan, 1981), dormancy characteristics (Heichel et al., 1981), fertility level (Collins and Duke, 1981), soil N content (Barnes et al., 1984), and soil moisture (Heichel et al., 1983). The effect of soil N on BNF has received much attention since it is necessary to consider this factor when selecting for improved alfalfa populations (Teuber and Phillips, 1988). Laboratory studies suggest that a nitrate concentration in growth media greater than 5 mM inhibits nodulation in legumes (Streeter, 1988). The nitrate concentration of the soil solution, which ranges from 2-400 mg 1-1 in agri- cultural soils, is therefore often high enough to inhibit nodulation (Barber, 1984; Barnes et al., 1984). BNF contributes less to the N needs of alfalfa in the beginning of the establishment year than in subsequent harvests and years. Heichel et al. (1981) examined BNF during the seeding year using the 15N isotope dilution method for BNF determination. BNF, on a per plant basis, increased about four-fold from the first to the third harvest and then declined to almost nil as the plants approached dormancy. The percent of plant N derived from BNF ranged from a minimum of 25-30% at first and fourth harvest to a maximum of 59-69% at second and third har- 9 vests. In a later study, Heichel et al. (1984) examined the inter-annual and intra-annual BNF in two cultivars in The varia- a four year alfalfa stand on a silt-loam soil. tion among harvests in the first year was similar to that observed in the earlier study (Heichel et al., 1981) with a seasonal average of 59% of crop N derived from BNF (168 kg N ha-1). In years three and four, BNF did not show significant intra-annual variation. This was largely due to the increased level of BNF at the first harvest, in contrast to the seeding year where it was significantly lower at the first harvest than at later harvests. The seasonal average portion of crop N derived from BNF increased to 61% (167 kg N ha-1) in year three and 77% (198 kg N ha-1) in year four. Wivstad et al. (1987) also reported a lower portion of N from BNF in the first harvest interval relative to later harvests. In a two year study in Sweden, seasonal BNF in an established alfalfa stand was determined using 15 N and an in situ acetylene reduction technique. The proportion of N derived from BNF was 70% (242 kg N ha-1) in year one and 80% (319 kg N ha-1) in year two. Lower BNF in the period after establishment reflects the time required for developing active nodules, which is typically from two to four weeks (FAO, 1984). The time requirement for nodulation and the degree of nodulation 10 are greatly affected by the presence of combined N (nitrate-N or ammonium-N), particularly nitrate. The level of nitrate in the soil solution from mineralization is a function of soil temperature and moisture (Greenwood, 1986). Thus, soil temperature may interact with soil N availability in regulating the nodulation of alfalfa during establishment. The Effect of Combined Nitrogen on BNF in Alfalfa A large number of reports have addressed different aspects of how combined N affects BNF in legumes (Streeter, 1988). In soils, ammonium-N is converted relatively quickly to nitrate-N by nitrification. Thus, most re- search has focused on the effect of nitrate on BNF in legumes. Nodulation of alfalfa is highly sensitive to nitrate. Nitrate application appears to inhibit the infection process in alfalfa by a decrease in root hair formation, a decrease in the number of infection threads, and an increase in the number of aborted infection events (Truchet and Dazzo, 1982; Munns, 1977). The effect of mineral N on infection is reflected in changes in nodule number. In general, as nitrate concentration in the surrounding media 11 exceeds about 5 mM, the number of nodules per plant decreases in many legumes (Dazzo and Brill, 1978). In alfalfa, there was a decline in nodule number at about 11.5 mM nitrate-N relative to a nil-N control (Heichel and Vance, 1979; MacDowall, 1982). Most soils have at least 2 mM nitrate in the soil solution (Barber, 1984). This suggests most soils contain enough nitrate-N to inhibit nodulation in alfalfa. However, most studies to examine the effects of nitrate-N on nodulation have been conducted in artificial media and at optimal growth temperatures (20-25°C). These results may not be directly applicable to field conditions where temperature, moisture, and nitrate levels are more variable. Other studies show low concentrations of N may increase nodulation of alfalfa (MacDowall, 1982; Fishbeck and Phillips, 1981; Richardson et al., 1957). The addi- tion of 0.3 mM nitrate-N to a vermiculite-gravel mix resulted in greater nodule number and weight than at nilN. Alfalfa grown at 2 mM N (as ammonium nitrate) had greater nodulation than a nil-N control (Fishbeck and Phillips, 1981). In a study with three alfalfa varieties, two of three varieties grown in a 0.03 mM nitrate-N solution had more nodules/plant than nil-N controls (Richardson et al., 1957). Differences in the effects of low levels of N on 12 nodulation likely reflect differences in rooting media, form of N, and time of application. Roots grown in solu- tion culture may grow and respond differently than roots developing in a solid media (Greenwood, 1986). Nitrate in solution culture may be more readily available for uptake than in a solid media such as vermiculite or soil. This suggests that alfalfa grown in solution culture would be sensitive to N inhibition of nodulation at a lower concentration than in soil (Heichel et al. 1981; Fishbeck and Phillips, 1981). Effects of N on nodulation depend on the form of N applied. Alfalfa growing in sterile sand culture supple- mented with an N solution containing 0.75 mM ammonium-N resulted in more nodules/plant than with nil-N or nitrateN of a comparable concentration (Richarson et al., 1957). However, the conversion of ammonium to nitrate by nitrification, which proceeds relatively rapidly in soils, does not occur in sterile conditions. Thus, the effects of nitrate in laboratory studies may be more applicable to field conditions. The timing of N application also affects results. A stimulative effect of N on nodulation was observed when N treatments were applied to inoculated, rooted cuttings (Fishbeck and Phillips, 1981). When N treatments are applied at seeding, low concentrations of nitrate general- 13 ly inhibit nodulation (Heichel et al., 1981; Richardson et al. 1957). A further consideration in evaluating nitrate effects on nodules is the indeterminate growth habit of alfalfa nodules. Alfalfa nodules can regrow, even after exposure to nitrate, once the nitrate concentration is lowered (Becana and Sprent, 1987; Vincent, 1977). This provides one explanation as to why established alfalfa has a greater tolerance to higher nitrate levels. Differing experi- mental conditions indicate that caution must be used when extrapolating laboratory results to the behavior of alfalfa in the field. In contrast to the infection response, the response of nodule mass per plant is often initially positive. Low concentrations of nitrate N (< 5 mM) in nutrient solutions result in an increase in nodule mass per plant, plant growth, and N content (Rawsthorne et al., 1985; Das, 1982). In fact, there is substantial evidence that some combined N is required for maximum growth and nodule formation of legumes (Rawsthorne et al., 1985; Eaglesham et al., 1983; Das, 1982). In certain circumstances, legumes that have received low amounts of combined N as nitrate may fix more N2 than plants receiving no additional nitrate (Eaglesham et al., 1983; Gibson, 1974). This is explained by the need for small amounts of inorganic N 14 for the formation of functional nodules during early seedling growth. Although some N is available from seed reserves, this may not be adequate to meet plant N demands. For example, Sprent and Raven (1985) estimated that nearly one-third of the seed N reserves in Phaseolus vulgaris may be required for nodule formation. In addi- tion, the development of functional nodules may require significantly more N than an equivalent amount of functional roots. Thus, it is possible that early seedling growth of some legumes is N limited. The Use of Preplant N in Alfalfa Production Preplant N fertilization of alfalfa sometimes increases dry matter yield in the establishment year. It appears that the effect depends largely on soil N content. Weed control and effective inoculation are also important factors (Peters and Stritzke, 1970). The rationale behind the effect is that although nodulated alfalfa meets most of it's N requirements from BNF, mineral N is required for early growth and nodule development. Studies have been conducted to determine if additional N during this prenodulation phase increases growth and nodulation and leads to increases in dry matter yield in the establishment year. 15 Recommendations for the use of preplant N in alfalfa are based on reports that low levels (<80 kg ha-1) of preplant N increase yield of first-cut alfalfa (Peters and Kelling, 1989; Peters and Stritzke, 1970; Ward and Blaser, 1961). Results from a series of studies in Wisconsin (Peters and Stritzke, 1970; Simson et al., 1981; Peters and Kelling, 1987 and 1989) suggest that the N supplying capacity of the soil, pH, and weed control are important factors in determining the response of N to preplant N. Peters and Stritzke (1970) attempted to minimize the effect of weeds with an application of EPTC (ethyl N,Ndipropylthiocarbamate). They planted three varieties and applied 95 kg ha-1 preplant N as ammonium nitrate in each of three establishment planting experiments. determined at first bloom. Yield was In two out of three years, the addition of N without EPTC significantly reduced the density of the alfalfa stand, compared to plots treated with EPTC with or without N. The reductions were attrib- uted to increased weed growth resulting from additional N, and not to direct fertilizer injury. Treatment with EPTC and N increased average alfalfa yields in one of the three years. Averaged across varieties the increase in dry matter was 300 kg ha-1, compared to EPTC with no N. The variety 'Vernal' showed the largest individual increase, about 900 kg ha-1. One reason given for the ambiguity of 16 the results is that while the EPTC did a reasonable job of controlling grassy weeds, it may have injured alfalfa in some plots. The results suggest that if weeds can be effectively controlled without injuring alfalfa, yield increases may result. Peters and Kelling (1987) applied from 10-40 lbs acre -1 -1 (11.2-44.8 kg ha -1 preplant N as either diammonium sulfate or calcium nitrate. They reported a first cut yield response in one year out of two, but only at the highest rate of diammonium sulfate. A yield increase in response to small amounts of N (22.4-44 kg ha-1) was sug- gested when soils contain less than 15-20 tons acre-1 organic matter. This would be equivalent to less than 0.1% total soil N, assuming that organic matter contains 5% N and that an acre-furrow-slice of soil weighs two million pounds. In a later study, Peters and Kelling (1989) examined the premise that N effects may be greater under acid soil conditions in low organic matter, light-textured soils. At the Marshfield, WI location, low, medium, and high pH treatments were 5.0, 6.2, and 6.9, respectively. Preplant N was applied as diammonium sulfate at 0, 20, 40, and 80 lbs N acre acre -1 -1 (0, 22.4, 44.8, 89.6 kg ha (44.8 kg ha -1 ) ammonium nitrate. -1 ) and as 40 lbs N Significant in- creases in first cut yields were reported for each addi- 17 tional increment of N fertilizer. Yield increases were observed within all pH treatments; however, the greatest proportional increase occurred in the low pH range. In the low pH soil, preplant application of 80 lbs N acre-1 (89.6 kg N had) more than doubled the yield, compared to 0 N. Similarly, yield was increased by preplant applica- tion of 40 lbs N acre-1 (44.8 kg ha-1) ammonium nitrate within all pH treatments, with the largest proportional increase in the low pH treatment. As in other studies, there was a greater percentage of weeds in the stand at the higher rates of preplant N. Alfalfa yields are not always increased by preplant N fertilization (Eardly et al., 1985; Simson et al., 1981; Washko and Price, 1970). Applying N at the time of seed- ing did not result in a consistent yield response in alfalfa grown on a silt loam soil (Simson et al., 1981). The lack of a response could have been due to the ability of the soil to supply considerable amounts of mineralized N for plant growth and nodule development. Results of studies on lower-organic matter soils support this concept (Peters and Kelling, 1987). An increase in alfalfa dry matter yield in response to N may be an indication of poor nodulation or ineffective nodules (Lee and Smith, 1972). In a three year field experiment, Eardly et al. (1985) examined the effect of 18 preplant N on yield and nitrogen fixation of effectively and ineffectively nodulated alfalfa in western Oregon. Preplant N was applied as ammonium nitrate at 0, 45, 90, 134, 179, and 224 kg ha-1 to a silt loam soil (fine-silty, mixed, mesic Aquultic Argixeroll) that contained an average of 8-12 mg kg -1 of available soil N. An increase in dry matter yield and N concentration was observed only in ineffectively nodulated plants. In both ineffectively and effectively nodulated plants, increasing rates of N application resulted in a decrease in nodulation and BNF, measured by acetylene reduction. Similar results were reported by McAuliffe et al. (1958), who established an alfalfa-tall fescue mixture in a sandy loam soil containing about 1 lb acre-1 (1.1 kg ha-1) inorganic N and 0.8% organic matter. Immediately after seeding, 15 N labeled diammonium sulfate was added at 0, 20, 40, and 80 lbs acre .1 N (0, 22.4, 44.8, 89.6 kg ha -1 ). BNF in seedling alfalfa, as measured by the 15N isotope dilution technique, decreased in proportion to increasing rates of N. Dry matter yield of tops was not significantly increased in either 6-week old or 10-week old seedlings. The lack of response to preplant N sometimes observed is often explained by differences in the N-supplying ability of the soil, pH, extent and effectiveness of nodulation, and weed control (Peters and Kelling, 1987; 19 Peters and Strizke, 1970). Consideration of these factors is essential in comparing results of field studies on the effect of N on alfalfa yield. The Effect of Temperature on Growth of Alfalfa The rate of germination and growth of alfalfa are affected by temperature. How, and to what extent, these processes are affected depends on the interaction of temperature with the genetic makeup of the plant. Under field conditions, temperature effects may be confounded with high light intensity and moisture stress. Given the relative difficulty of controlling these factors in the field, many studies on the effects of temperature on alfalfa growth and BNF have been conducted under greenhouse conditions or in growth chambers. Alfalfa will germinate at temperatures as low as 2°C and as high as 40°C (Stone et al., 1979; McElgunn, 1973). The optimum range, assuming other factors are conducive to germination, is 19-25°C (Fick et al., 1988). Germination is very sensitive to moisture stress, and temperature responses in the field can be greatly modified by soil moisture status (Triplett and Tesar, 1960). When adequate water is available, the rate of germination is temperature dependent. The rate of germination increases until about 20 27°C and then decreases sharply at about 30°C. Despite differences in rate of germination, the percent germination after one week differs little between 5°C and 35°C Thus, although warmer (Townsend and McGinnies, 1972). temperatures may promote earlier germination, with adequate time and water availability, a successful stand may be established at temperatures higher or lower than the optimal range. Temperature also plays a key role in seedling development. Growth of recently germinated alfalfa seedlings is most rapid at 20°C to 30°C (Hesterman and Teuber, 1981). There is a rapid decline in growth rate outside a temperature range of 10-37°C (Harding and Sheehy, 1980). Alfalfa development is hastened by increasing temperature and photoperiod (Fick et al., 1988, Jensen et al., 1967; Smith, 1970). Faster early growth may result in higher yields in first and second year alfalfa (Sibma and Spiertz, 1986). The relationship of root growth to shoot growth is also temperature dependent (Lie, 1974; Gist and Mott, 1957; Pearson and Hunt, 1972). The ratio of the rate of dry matter accumulation in the roots to shoots is over 50% higher in alfalfa grown at 15/10°C than plants grown at 35/30°C (Pearson and Hunt, 1972). In 45 day old alfalfa, the root/shoot dry weight ratio declined from 0.59 at 16°C 21 to 0.36 at 32°C (Gist and Mott, 1957). The root/shoot dry matter ratio of alfalfa varies curvilinearly with respect to time from emergence. The exact curvilinear function varies considerably across the temperature range of 15/10°C to 35/30°C. As a result, in early growth the root/shoot dry matter ratio at 15/10°C is smaller than that at 35/30°C (Pearson and Hunt, 1972). These results demonstrate that root/shoot dry weight ratio is a function of both growth temperature and growth stage. The Effect of Temperature on BNF in Alfalfa Temperature affects all aspects of legume infection, nodule formation, and BNF, with root temperature being more important than air temperature (Lie, 1974). There is a fairly broad temperature range over which these processes will occur, although each process may exhibit a different optimal temperature response (Roughley and Date, 1986). The legume/Rhizobium symbiosis can adapt to a wide range of soil temperatures. Nodulated legumes are ob- served under very cold (Ek-Jander and Fahraeus, 1971) and very warm (Tadmor et al., 1971) temperature regimes. Mod- ulation of temperate legumes may take place, albeit slowly, at temperatures as low as 7°C (Roughley, 1970). Once formed, nodules can fix N2 at temperatures as low as 2°C 22 (Day and Dart, 1971). Nevertheless, extremes in tempera- ture generally inhibit all key aspects of BNF in legumes, i.e. infection, nodule growth, and N2 fixing activity (Sprent and Sprent, 1990; Dart et al., 1975). Nodulation is delayed at cool temperatures. At temperatures below 15°C, alfalfa nodulation is inhibited (Jones and Tisdale, 1921; Duke and Doehlert, 1981). Maximum acetylene-reduction activity in alfalfa occurs from 20-25°C (Waughman, 1977). Nodules generally contain less bacteroid tissue at low temperatures, with a reduction in efficiency of BNF (Lie, 1974). In sub-clover, the greatest amount of bacteroid tissue is formed at 11°C, but the highest BNF efficiency per unit of bacteroid tissue occurs at 19°C (Roughley, 1970). Higher soil temperatures appear to reduce both nodulation and BNF. In isolated Phaseolus roots, nodulation was reduced by about 70% at 30°C relative to 25°C. Howev- er, a temperature conditioning effect was also observed. Roots exposed to 25°C for at least 3 days after inoculation and then placed in 30°C nodulated as much as roots held at a constant 25°C. Nodulation was completely inhib- ited at 12°C and 33°C (Barrios et al., 1963). The impact of temperature stress is modified by the timing of the stress. A cultivar of pea (Pisium sativum cv. Iran) fails to nodulate when exposed to a root temper- 23 ature of 18-20°C. However, if exposed to a temperature of 25°C for 24 hours within the first 3 days after inoculation, nodulation occurs. If the temperature is then lowered to 20°C, nodule growth and BNF proceed normally (Lie, 1971). It appears that the first few days after inoculation is a critical period during which a temperature greater than 20°C is required to initiate infection. Thereafter growth and activity of nodules is less sensitive to temperature. It may be that a similar relation- ship between temperature and nodulation exists in alfalfa, although apparently this has not been determined. Methods of Measuring Biological Nitrogen Fixation Measurement of BNF is controversial (Sprent and Sprent, 1990; Smith et al., 1987). The difficulty is distin- guishing the portion of the total plant N that was derived from atmospheric N2, as opposed to soil or fertilizer derived N. Most procedures used are modifications of three general methods: 1) the acetylene reduction method, 2) the 15N isotope dilution technique (IDT), and 3) the difference method (DM). The relatively simple, rapid, and inex- pensive nature of the acetylene reduction assay prompted an large increase in BNF research after it's introduction (Sprent and Sprent, 1990). A variety of modifications 24 have been developed in an effort to facilitate it's use and improve the accuracy of the results obtained (Criswell et al., 1976; Medereski and Streeter, 1977; Eardly et al., 1985). In the basic procedure, attached or detached roots and/or nodules are exposed to an air-acetylene mixture and incubated for a period of time ranging from a few minutes to as long as one or more hours. A subsample of the mixture is injected into a gas chromatograph to determine the quantity of acetylene reduced to ethylene by nitrogenase. The amount of acetylene reduced is assumed to be proportional to the rate at which N2 would be reduced to NH3 The limitations of using detached root systems or nodules have been widely discussed. Intact plants exhibit from two to five times higher rates of acetylene reduction than detached systems (Mague and Burris, 1972). In addi- tion, major problems arise from the usual practice of employing a closed assay system (Giller, 1987; Minchin et al., 1986). Studies with alfalfa, white clover, and peas demonstrate that there is a large decline in respiration rate in the presence of acetylene (Minchin et al., 1983; Haystead, et al., 1980; Minchin et al., 1982). Attempts to resolve these difficulties have led to the development of flow-though systems (Silvester et al., 1989), but criticisms of the technique persist (Gordon et al., 1989). 25 There appears to be a consensus that BNF is more accurately estimated with either the IDT or the DM (Heichel et al., 1989; Chalk, 1985; Witty, 1983), although each method has limitations (Hauch and Weaver, 1986; Witty, 1983). the IDT. 15 The DM is simpler and less expensive than N enriched fertilizer is expensive and the isotopic analysis of the resulting samples may be costly if large numbers of samples need to be analyzed. It has been reported, however, that the DM tends to underestimate BNF and is less precise than the IDT (Henson and Heichel, 1984; Ruschel et al., 1979). The Difference Method The simplest and most economical way of determining BNF in legumes is the DM. This method has become more attractive to researchers with the release of several nonN2-fixing legume control plants. The alfalfa BNF research group at University of Minnesota has registered and released four alfalfa germplasms selected specifically for use as non-N2-fixing controls in alfalfa BNF research (Barnes et al., 1988b). Henson and Heichel (1984) com- pared the agreement and precision of the 15N isotope dilu- tion technique (IDT) with the DM for determining BNF in alfalfa. An ineffectively nodulating alfalfa strain was 26 used as the non-fixing control plant. They concluded that non-fixing alfalfa was an acceptable control for BNF measurement by either the IDT or DM, with the caution that the DM probably underestimates BNF relative to the IDT. Therefore, the use of the DM may serve as a less-expensive substitute for the more expensive IDT, in cultivar evaluation and alfalfa management studies (Henson and Heichel, 1984; Heichel et al., 1989). The primary assumption underlying the difference method is that the N2-fixing plant and the non-N2-fixing plant assimilate identical amounts of soil N and that fertilizer N applied is also assimilated with equal efficiency (Chalk, 1985; Hauch and Weaver, 1986). In theory, the ideal non-N2-fixing plant would be one which is known to resemble closely the growth and rooting pattern of the N2-fixing plant. For this reason a non-nodulating isoline may be the most ideal non N2-fixing control plant (Heichel et al., 1989). According to Chalk (1985), 15N isotope studies support this assumption. The percent of the plant N that is derived from the atmosphere (% Ndfa) may be calculated from the following three equations in which DRM refers to whole plant or shoot dry matter, fp refers to the N2-fixing plant and nfp refers to the non-N2-fixing plant: (1) N yield = DRM x % N 27 (2) BNF = N yield (fp) - N yield (nfp) (3) % Ndfa = [BNF / N yield (fp)] x 100 It is preferable to use total DM (root + shoot dry matter yield) in the calculation, however given the difficulty of recovering root systems in the field, herbage samples are usually used to estimate N yield. The DM technique gives similar results in either low or high N soils (Henson and Heichel, 1984). However, if fertilizer N is applied it is recommended that rates be kept low due to the sensitivity of nodulation and BNF to soil nitrate (Ruschel et al., 1979). The relative simplicity and economy of the DM may make measurement of BNF more affordable in future studies. 28 III. THE EFFECT OF PREPLANT NITROGEN FERTILIZATION AND SOIL TEMPERATURE ON BIOLOGICAL NITROGEN FIXATION AND YIELD OF ALFALFA (Medicago sativa L.) Abstract The usefulness of preplant nitrogen (N) in establishing alfalfa under different environmental conditions has not been well characterized. This study was conducted to determine whether preplant N applications to alfalfa (Medicago sativa L. cv. 'Vernema') established in colder production areas significantly affects yield, percent N derived from biological nitrogen fixation (PBNF), and shoot N concentration in first cut alfalfa. Field experi- ments were conducted at Powell Butte, OR, on an Ayres sandy loam to determine the effect of preplant N on yield and shoot N concentration of first cut alfalfa. Growth chamber experiments were conducted to examine the effect of preplant N and soil temperature on yield, PBNF, and shoot N concentration of first cut alfalfa. In field experiments, preplant N application had no effect on shoot N concentration in either year. In 1987 there was no effect of preplant N on dry matter yield. Application of 20-40 kg ha-1 preplant N resulted in increased dry matter yields in 1988. In growth chamber experiments, at 18/12°C 29 and 24/16°C, 40 kg ha-1 preplant N resulted in increased shoot and root dry matter yield. Increasing rates of preplant N did not result in lower PBNF at any temperature. Shoot N concentration was not affected by preplant N treatments at any temperature. Preplant N may increase first cut yield under conditions of low soil temperature (<15°C) and low soil N availability (<15 ppm nitrate) without decreasing PBNF. Assessment of both soil tempera- ture and soil N availability is essential in determining the potential for a yield response to preplant N. 30 Introduction Alfalfa (Medicago sativa L.) is one of the most important forage crops cultivated in the U.S. (Barnes et al., 1988). The use of nitrogen (N) in the establishment of pure stands of alfalfa and other legumes is controverHojjati et al. sial. (1978) reported that 21 of 45 U.S. universities recommended N in establishment of pure legume stands. Three states had no recommendations and two had indefinite recommendations. The state of Oregon does not have a definite recommendation for the use of preplant N, also known as "starter N", in alfalfa establishment. Some results suggest that starter N does not result in increased yield (Eardly et al., 1985; Simson et al., 1981). In addition, starter N increases weed growth and may reduce stands (Ward and Blaser, 1961; Peters and Stritzke, 1970). In field studies, Eardly et al. (1988) reported that the application of up to 90 kg ha-1 preplant N did not result in significant dry matter yield increase in the first harvest of the seeding year. Other reports suggest that, if weeds are controlled, starter N may increase yield in the establishment year (Peters and Stritzke, 1970; Peters and Kelling, 1987; 1989). N sup- plying capacity of the soil, pH, inoculation, and weed control are important factors in determining the response 31 of alfalfa to preplant N. Differences in these factors may explain the different responses to preplant N. The application of combined N to legumes affects infection, growth, and activity of legume nodules (StreetThe relationship between BNF and mineral N, er, 1988). particularly nitrate, is often antagonistic. Some studies indicate, however, that combined N is required for maximum nodule growth and maximum BNF in legumes (Mahon and Child, 1979; Allos and Bartholomew, 1959). The specific conditions under which N applications will result in an increase in alfalfa yield are not well defined. The N-content of the soil and soil temperature are important factors (Minchin and Sprent, 1983; Barta, 1978). Specific information on how these factors affect yield and BNF is needed. The objective of these studies was to determine the effect of preplant N fertilization and soil temperature on the yield, shoot N concentration, and percent of plant N derived from BNF (PBNF) of first cut alfalfa. 32 Materials and Methods Field Experiments Field experiments were conducted in 1987 and 1988 at the Central Oregon Agricultural Experiment Station, Powell Butte, OR, on an Ayres sandy loam (loamy, mixed, mesic, shallow Xerollic Durargid), with 0-2% slope. This site was chosen because it is a low N soil and the soil temperature is cool (10-15°C) at planting. Twenty pre-treatment soil samples (five from each of four replicates) were taken on March 19, 1987 and March 20, 1988. These were then bulked into four samples for analysis. Soil NH4-N and NO3-N were extracted with 2 M KC1. NO3 and NH4 Concentration of in extracts was determined by a colorimetric procedure (Keeney and Nelson, 1982) in an Alpkem RFA 300 Autoanalyzer. Soil temperatures at 10 cm and 20 cm depth were obtained from records of the National Weather Service weather station located at Redmond, OR, about 12 km from the Powell Butte Experiment Station. A randomized complete block design consisting of four replications of five rates of NH4NO3 was used to provide N levels of 0, 10, 20, 40, and 60 kg ha-1. were 10 m X 2 m. Individual plots In 1987, 80 kg ha-1 P2O5 and 112 kg ha-1 S were applied pre-plant. In 1988, 154 kg ha-1 P2O5 and 100 33 kg ha-1 S were applied. Shortly before planting, an aque- ous solution of NH4NO3 was sprayed onto the plots using a small sprayer fitted with a 1 m wide boom. 'Vernema' alfalfa (Round Butte Seed Growers, Culver, OR), inoculated with a commerical peat based Rhizobium inoculum (Nitragin Co., Milwaukee, WI), using a sucrose solution sticker, was broadcast onto plots at 22 kg on June 18, 1987 and 25 kg ha-1 on May 27, 1988. ha-1 Follow- ing planting, the plots were irrigated and managed according to commercial practices for the area. ous weed infestation occurred. In 1987, seri- On July 31 a mixture of 0.2 kg a.i. ha-1 Buctril and 0.6 kg a.i. ha-1 2,4-DB was applied. Thereafter the plots were hand-weeded for con- trol of lambsquarter and redroot pigweed. In 1988, an unusually warm period in late July, combined with problems with the irrigation system, resulted in significant water stress on some plots, which hastened maturation. There- fore, the 1988 harvest occurred several weeks earlier than normally expected. Randomly selected shoot samples were taken three times during each growing season. In 1987 samples were taken on July 16, August 13, and August 27, and in 1988 on July 14, July 29, and August 16. The samples were dried at 70°C and ground in either a small spice grinder or a Wiley Mill (Arthur H. Thomas, Philadephia, PA) to pass a 34 40 mesh screen. Shoot N concentration was determined by a modified Kjeldahl method (Bremner and Mulvaney, 1982). Samples were digested using a CuSO4- Se -K2SO4 catalyst. Total NH4 in the digest was determined by a colorimetric procedure in an Alpkem RFA 300 Autoanalyzer (Alpkem Co., Portland, OR). Plots were harvested at the early bloom stage, as determined by visual inspection. This occurred on Septem- ber 10, 1987 and August 16, 1988. A self-propelled small plot forage harvester was used to cut a 6 m2 swath within each plot. A swing-arm mounted electronic balance enabled whole plot fresh weight to be determined immediately after cutting. Subsamples of herbage were collected, sealed in plastic bags, and removed to the experiment station where they were weighed and then placed in a 70°C drying oven. The dry samples were reweighed and the calculated percent moisture used to determine the dry matter yield for each plot. 35 Growth Chamber Experiments Growth chamber experiments were conducted in 1990-91 in growth chambers located on the Oregon State University campus, Corvallis, OR. Washed river sand (Corvallis Land- scape Supply, Corvallis, OR) was used to fill 10 cm diameter X 1 m long, capped PVC tubes. Seven 0.3 cm diameter holes were drilled in the end caps and covered with 2 cm gravel, to allow drainage. The river sand was analyzed m2 m-2 and 0.4 g B as H3B03, 4 g in S as CaSO4, 6 g P as CaHPO4, and 12.5 g m-2 K as KC1 were added to assure proper mineral nutrition. A mix of incandescent and fluorescent tubes provided an average PPFD of 350-450 s . mole photon M-2 The photoperiod was 16h day. A split-plot design was used for the growth chamber experiments with three day/night temperature regimes (27/21°C, 24/16°C, and 18/12°C) as the main plots, and five preplant N rates (0, subplots. 1, 2, 4, and 8 g m-2 N as NH4NO3) as Four replicates (two tubes per replicate) were used to insure adequate material for sampling. N treat- ments were applied as 50 ml aqueous solutions. After applying N treatments, 100 mg per pot of certified 'Verne- ma' alfalfa seeds were pressed into the soil surface and covered with a thin layer of a peat based Rhizobium inoculum and soil. 36 Seeds of ineffectively nodulating 'Saranac' alfalfa (courtesy of D.K. Barnes, USDA-ARS, Univ. of Minnesota, St. Paul, MN) were planted in separate tubes as non -N2fixing controls. Earlier experiments had demonstrated that these plants would require additional N to grow in the river sand. Therefore, 80 kg ha-1 N was added to the ineffective 'Saranac' pots before planting. Four replicates of plant samples were collected by removing 6-10 whole plants at approximately the early vegetative, early bud, late bud, and early flowering growth stages (final harvest) as determined by the mean stage by count (MSC) method (Fick and Mueller, 1989). Thermal time in growing degree days (GDD) was calculated according to the equation GDD = E ([Tum + Tmin / 2) - Tb) where Tom is the daily maximum temperature, Trnib is the minimum daily temperature, and Tb is the base temperature. The most common Tb used for alfalfa is 5°C (Sharratt et al., 1989) and was used for GDD calculations. Whole plant samples were collected by carefully digging out individual plants with a small spoon and washing roots with water periodically to loosen and clean them. By digging down around the roots about 12-16 cm and moving the root back and forth, the large tap root could be removed largely intact from the pot. The weight of the large tap root is 37 assumed to account for most (>90%) of the total root weight. Roots and shoots were dried at 70°C. The dry samples were weighed and ground in either a small spice grinder or a Wiley mill to pass a 40-mesh screen. Total N concentration was determined by a modified Kjeldahl N procedure as described under field experiments. Total N yield was calculated as the product of dry weight and N concentration. The percent of plant N de- rived from the atmosphere (PBNF) was determined by the difference method as described by Henson and Heichel (1984) and Heichel et al. (1989), according to the equations: (1) BNF = N yield (fp) N yield (nfp) (2) PBNF = [BNF / N yield (fp)] x 100 in which fp refers to the N2-fixing plant and nfp refers to the non-N2-fixing plant. This procedure assumes that the N2-fixing plant and the non-N2-fixing crop assimilate equal amounts of soil and fertilizer N and that the nonN2-fixing plant develops root and shoot systems similar to the N2-fixing line. In our experiments, the fixing and non-fixing lines were not identical in growth habit. Also, as noted above, additional fertilizer N was required to maintain vigorous growth of the non-fixing 'Saranac'. Therefore, the calculations of PBNF presented herein are subject to error to the degree that the non-fixing line 38 departed from the pattern of N-uptake in the fixing line. Thus, the data presented is to be used for treatment comparisons rather than quantitatively. Statistical Analysis Data were analyzed using the SAS microcomputer software system (SAS, 1990). In the field experiments a one way analysis of variance (ANOVA) for combined years data showed significant differences between years with respect to shoot N, but not for dry matter yield at harvest. Therefore an ANOVA for shoot N was conducted separately for years. Significance of differences between treatment means were computed by Duncan's multiple range test (SAS, 1990). In the growth chamber experiments the PROC ANOVA and PROC GLM procedures were used to examine differences between temperature treatments, N treatments and temperature X N interaction effects with respect to shoot and root dry weight at harvest, shoot N concentration, and PBNF. The PROC STEPWISE and PROC REG procedures were used to determine the best fitting linear regression relating shoot dry matter and N accumulation to cumulative thermal time. 39 Results and Discussion Field Experiments The physical and chemical characteristics of the soil at the Powell Butte experiment site are summarized in Table III.1. Relative to agricultural soils in the U.S. the values for nitrate are low (Barber, 1984). The aver- age 10 cm soil temperatures during the 2 weeks following planting was 18°C in 1987 and 10°C in 1988. Shoot N concentration declined in both years as the plants matured, probably reflecting dry matter dilution (Table 111.2). Shoot N concentration within sampling dates was not affected by N treatments. et al. Similarly, Eardly (1988) reported no increases in N concentration in 10 week old field grown as preplant N rates increased from zero to 90 kg ha-1. In an earlier experiment (Eardly et al., 1985) shoot N concentration increased only at N rates from 90-224 kg ha-1. There were no differences in dry matter yield among N treatments in 1987 (Table 111.3). In 1988 the yield of the highest preplant N treatment was significantly lower than at 20 and 40 kg ha-1 N. Visual inspection of the field plots about one week before harvest revealed serious water stress on a number of plots due to difficulties with Table 111.1. Soil characteristics of field site at Powell Butte, OR and washed river sand used in growth chamber experiments before treatments applied. Soil pH P K B mg /4144. NO3 Mg Ca cmol kg kg-1 -1 1987 Field t 6.6 14 261 0.4 4.2 5.3 10.1 4.0 1988 Field 6.6 17 288 0.5 2.4 17.0 10.8 4.4 River Sand t 6.7 22 238 0.3 2.2 6.1 6.9 3.4 t Mean of four samples taken to a depth of approximately 15 cm. t Mean of six samples. Table 111.2. Shoot N concentration of alfalfa grown at five levels of preplant N at Powell Butte, OR in 1987 and 1988. Preplant N 1987 1988 Days after planting Days after planting 28 56 70 48 63 81 g N kg- kg ha-1 41t 37 38 39 32 27 10 43 38 40 37 32 27 20 45 35 36 34 26 23 40 45 35 36 36 31 27 60 45 34 36 38 33 26 44a1 36b 36b 37a 30b 26c 0 Means t Means were not significantly different within dates at P=0.05. : Date means followed by different letters are significantly different at the 0.01 level as determined by Duncan's Multiple Range Test. 42 Dry matter yield of alfalfa grown at five Table 111.3. levels of preplant N at Powell Butte, OR in 1987 and 1988. Preplant N 1988 1987 Mg ha-1 kg ha-1 0 7.1 3.0 10 6.0 6.4 20 6.5 14.8 40 6.6 8.6 60 6.6 2.6 N.S. 4.3 LSD(0.05) 43 irrigation. Because of the uneven slope of the plots, the effects were not localized entirely in one part of the field or in a distinct pattern that would facilitate covariance analysis. Thus, it is possible that the low yield at the highest N level reflects the effects of water stress and not a negative effect of preplant N. It seems unlikely, though, that the large increases in dry matter yield observed at 20-40 kg N ha-1 are an artifact of irrigation effects. The range in dry matter yield of replicates at 20 kg N ha-1 was 11.6-18.1 Mg ha-1 compared to 0.7-12.9 Mg ha-1 for all other N treatments. Therefore, an increase in yield was obtained at 20 and 40 kg ha-1 N relative to zero N. The 1987 and 1988 experiments were conducted in adjacent fields and the soil nitrogen content was similar in both years. Therefore, aside from the difficulties with the irrigation system previously mentioned, the only measured factor that may explain the differences in yield response between years is the difference in soil temperature during seedling development. The average 10 cm soil temperature during the 2 weeks following planting was 18°C in 1987 and 10°C in 1988. The lower soil temperature in 1988 was well below the optimal temperature for nodulation of alfalfa (18°C) as reported by Jones and Tisdale (1921). N available from mineralization would also be reduced (Greenwood, 1986). In 1988, the readily available fertilizer N may have provided additional N during a period when mineralizable N and BNF could not meet the plant N demand. Other researchers have proposed that an N deficit may develop during the period of nodule development in alfalfa when the N demand of the plant can not be met by either BNF or soil N (Peters and Kelling, 1987; Fishbeck and Phillips, 1981). The deficit would be exacerbated by 44 soils with low N-supplying capacity (Peters and Kelling, The increase in dry matter yield in 1988 implies that the potential for this N deficit is increased when 1989). soil temperatures are below optimum for BNF and soil N mineralization. An additional concern with the use of preplant N is the promotion of weed growth. In both years of the field study, the addition of preplant N increased weed growth and necessitated herbicide application. However, in 1988 weeds were controlled earlier and the weed infestation was much smaller, which may have contributed to higher yields at 20-40 kg ha-1. Peters and Strizke (1970) noted that when weeds were controlled without injury to alfalfa, N application resulted in yield increases in two out of three cultivars. Lee and Smith (1972) suggested that a yield response of legumes to fertilizer N may simply reflect poor nodulation or ineffective nodules. This apparently assumes that most of the plant N demand during early growth is provided by BNF. In field experiments with alfalfa, however, Heichel et al. (1981) observed that about 25-30% of plant N was derived from BNF during the period preceding the first harvest. Thus, even with effective nodulation, considerable amounts of mineralized or fertilizer N is required to meet the plant demand for N in the period preceding first harvest. In the 1988 experiment, visual inspection of plants demonstrated the presence of nodules at all N levels within five weeks after planting. In addition, neither shoot N concentration (Table 111.2) nor plant color suggested N stress at any time during the season. Thus, the use of preplant N may result in in- creased yield in low N soils when soil temperature is cool and weeds are controlled. 45 Growth Chamber Experiments Dry Matter Yield Combined analysis of variance of the growth chamber experiments indicated that temperature, N, and the temperature X N interaction had significant effects on shoot dry matter yield (Table 111.4). Dry weight declined slightly as root temperature increased from 18/12°C to 24/16°C, and then decreased sharply at 27/21°C (Table 111.5), possibly as a consequence of increased respiration relative to photosynthesis (Fick et al., 1988). N treat- ments within temperatures had the largest effect on dry weight at 18/12°C. The largest dry matter yield was observed at 40 kg ha-1 in both the 18/12°C and 24/16°C soil temperature treatments. At 24/16°C and 27/21°C, the yield of the control was equal to the yield at the highest rate of preplant N (80 kg ha-1). Linear regression of seasonal shoot dry matter accu- mulation versus thermal time (GDDO is presented in Table 111.6. At 24/16°C (r2=0.75) and 27/21°C (r2=0.91) soil temperatures, GDD5 was a reasonably good predictor of shoot dry matter accumulation independent of preplant N rate. At 18/12°C, a linear regression equation containing both GDD5 and preplant N was a better predictor (r2=0.70) Table 111.4. Combined analysis of variance of three day/night soil temperature regimes and five levels of preplant N in growth chamber experiments. Mean squares PBNF % Shoot N 135.6 0.01 304.8** 0.04 0.6 24.8 0.02 0.7** 1.2** 83.2t 0.02 0.6** 1.0** 76.1 0.03 0.2 0.3 35.0 0.02 Source of variation df Total 58 Block 3 0.3 Temperature 2 7.2** Block*Temperature 6 0.2 N level 4 Temperature*N level 8 Error 35 Shoot DM Root DM 0.9 28.7** *, ** Significant at the 0.05 and 0.01 probability levels, respectively. t Significant at the 0.07 probability level. 47 Table 111.5. Shoot dry matter yield at harvest of alfal- fa grown in three soil temperatures and five levels of preplant N in growth chamber experiments. Day/night soil temperature Preplant N 18/12°C kg hdl 24/16°C 27/21°C g plant-1 1.6±0.2t 1.4±0.3 0.8±0.1 10 1.2±0.3 1.6±0.1 0.8±0.1 20 1.8±0.4 1.4±0.1 0.9±0.2 40 2.7±0.8 2.0±0.7 0.7±0.1 80 2.5±1.1 1.5±0.4 0.8±0.1 0 t Values are means of four replicates ± standard deviation except for the 0 N treatment for 18/12°C which is the mean of three replicates. Table 111.6. Linear regression analysis of shoot dry matter accumulation (SHDW) in response to thermal time (GDD5) and preplant N in growth chamber experiments. Soil Regression equation 2 F:GDD5t F:N 12.9** 2.3*t 0.70 r temperature 18/12°C SHDW = 0.9 X 10 -3 GDD5 + 9.4 X 10 24/16°C SHDW = 1.1 x 10 -3 GDD5 - 0.97 15.1** NS 0.75 27/21°C SHDW = 5.0 X 10 -4 GDD5 - 0.60 28.4** NS 0.91 -2 N - 0.79 t GDD5 is the cumulative growing degree days with a base temperature of 5°C. 49 of shoot dry matter accumulation than GDD5 alone. Shoot dry matter accumulation rate was similar at 18/12°C (1.1 X 10 -3 g GDD5 -.1 ) and 24/16°C (0.9 X 10 -3 -1 g GDD5 ). At 27/21°C, shoot dry matter accumulation rate (5.0 X 104 g GDD5 -1) was reduced by 55%. Smith (1970) also reported a decline in shoot dry weight in alfalfa when growth temperature was increased from 15/10°C to 27/21°C. More rapid early growth at cooler temperatures, i.e. less than 24/16°C for 'Vernema', would deplete N from seed reserves more rapidly. The result would be higher demand for soil available N than at warmer temperatures. This may explain the yield response to preplant N at the coolest soil temperature. Temperature, preplant N, and temperature X N interaction had significant effects on root dry weight (Table 111.4 and 111.7). The largest effects were observed at 18/12°C, in which root dry weight increased with increasing rates of preplant N application, with the maximum yield at 80 kg ha-1 (Table 111.7). Similar results were reported by MacDowell (1982) with alfalfa grown at 25/20°C, in which root dry weight increased in response to added N up to 15 mM. Root growth at 27/21°C was reduced and confirms that the highest soil temperature regime was above the optimum for root dry matter accumulation for 'Vernema' alfalfa. 50 Table 111.7. Root dry matter yield at harvest of alfalfa grown in three soil temperatures and five levels of preplant N in growth chamber experiments. Day/night soil temperature Preplant N 18/12°C 27/21°C g plant-1 kg ha-1 2.5±0.3t 1.3±0.3 0.6±0.2 10 2.1±0.3 1.4±0.2 0.7±0.1 20 2.7±0.6 1.3±0.1 0.7±0.2 40 3.4±1.0 1.6±0.3 0.7±0.1 80 4.3±2.0 1.4±0.2 0.6±0.1 0 t 24/16°C Values are means of four replicates ± standard deviation except for the 0 N treatment for 18/12°C which is the mean of three replicates. 51 Combined N has been shown to increase dry matter yield in alfalfa depending on the amount and time of application in greenhouse and growth chamber studies (Fishbeck and Phillips, 1981). Heichel and Vance (1979) noted an increase in dry weight in response to added N in nodulated alfalfa grown at 27/19°C in solution culture for 14 days. 1 ) Increasing N from zero to 3.5 mM (50 mg kg in their experiments resulted in a 60% increase in average whole plant dry weight. Nodule number, however, was reduced by about 40% over the same range of N. In this relatively short growth period, the availability of higher amounts of inorganic N resulted in greater dry matter accumulation than when BNF was the sole source of N. Available N, soil type, and legume species are important factors in determining the dry matter accumulation response of legumes to added N (Peters and Kelling, 1989; Fishbeck and Phillips, 1981; Hojjati et al., 1978). In addition, the interactive effect of temperature and nitrate level on dry matter accumulation has been reported for white clover (MacDuff et al., 1989). The strong significance of the temperature and preplant N interaction (Table 111.4) indicates this is also true for dry matter accumulation in alfalfa grown in a low N soil. 52 PBNF and N Accumulation Both temperature and the temperature X N interaction had significant effects on PBNF (Table 111.4). Average PBNF was similar at 24/16°C and 27/21°C, but generally higher at 18/12°C (Table 111.8). PBNF was not reduced by increasing preplant N at any temperature. At 18/12°C, 80 kg N ha-1 resulted in a 14% increase in PBNF. BNF is profoundly affected by root temperature and is closely related to changes in dry matter accumulation (Gordon et al., 1989; MacDuff et al., 1989). Changes in PBNF in response to temperature have been shown to parallel changes in dry matter accumulation in alfalfa (Wery et al., 1986) and other legumes (Gates and Silsbury, 1987; Date and Roughley, 1986). This relationship was con- firmed in our growth chamber experiments (Tables 111.5, 111.8). Linear regression of shoot N accumulation in response to GDD5 and preplant N is presented in Table 111.9. The rate of N accumulation was similar at root temperatures of 18/12°C (2.1 X 10-3 g N GDD5-1) and 24/16°C (2.6 X 10-3 g N GDD5-1) g N GDD5-1). , but reduced at 27/21°C (1.1 X 10 -3 At 18/12°C, the best fitting linear regres- sion included a preplant N component. Shoot N concentration was unaffected by N treatments (Table 111.10). 53 Table 111.8. Percent N derived from biological nitrogen fixation (PBNF) in alfalfa grown in three soil temperatures and five nitrogen levels in growth chamber experiments. Day/night soil temperature Preplant N 18/12°C 24/16°C 27/21°C PBNF kg ha-1 73±2t 64±10 67±9 10 68±6 71±3 72±3 20 75±5 66±5 72±6 40 81±7 73±5 70±5 80 83±6 65±11 71±5 Means 76a4 68b 70b 0 t Values are means of four replicates ± standard deviation except for the 0 N treatment for 18/12°C which is the mean of three replicates. : Temperature means followed by different letters are significantly different at the 0.01 level as determined by Duncan's Multiple Range Test. Table 111.9. Linear regression analysis of shoot N accumulation (SHN) in response to thermal time (GDD5) and preplant N in growth chamber experiments. Soil Regression equation 2 F:GDD5t F:N r 11.8** 3.2* 0.67 2.15 14.9** NS 0.74 GDD5 - 1.38 26.8** NS 0.90 temperature 18/12°C SHN = 2.1 X 10 24/16°C SHN = 2.6 x 10 -3 27/21°C SHN = 1.1 X 10 -3 GDD 5 + 3.1 X 10 GDD5 N - 2.43 t GDD5 is the cumulative growing degree days with a base temperature of 5°C. 55 Table 111.10. Shoot N concentration at harvest of alfal- fa grown in three soil temperatures and five levels of preplant N in growth chamber experiments. Day/night soil temperature Preplant N g m 18/12°C -2 24/16°C g N 27/21°C kg-1 21±1t 20±1 22±1 10 20±1 22±2 21±1 20 20±1 23±2 22±2 40 21±2 22±2 22±1 80 22±1 21±1 23±2 0 t The effects of temperature, N level, and temperature X N level interaction were not significant at P=0.05. Values are means of four replicates ± standard deviation except for the 0 N treatment for 18/12°C which is the mean of three replicates. 56 In white clover grown in solution culture with 10 mM nitrate, PBNF decreased from 29% at a root temperature of 17°C to 11% at 25°C (MacDuff et al., 1989). In our study, PBNF also declined at higher temperatures, from 64% at 18/12°C to 45% at 27/21°C (Table 111.8). PBNF was highest at 18/12°C, despite reports that alfalfa nodulation is inhibited at soil temperatures below 15°C and Tisdale, 1921). (Jones The warmer daytime temperatures may have eliminated negative effects of the cool night temperatures. At 18/12°C, the addition of fertilizer N up to 80 kg ha -1 . did not result in a decline in PBNF, despite the well documented negative effects of combined N on BNF (Streeter, 1988). Most of those studies, however, have been conducted at average day/night soil temperatures above 20°C. Studies with field-grown alfalfa by Wery et al. (1986) suggested that when a lack of N was limiting growth, the addition of a small amount of N (35 kg ha-1 N) favored fixation instead of nitrate assimilation. The additional N also resulted in a greater accumulation of N and dry matter. In the field, root temperature in the spring will often remain below shoot temperature during early plant development. During this stage of relatively rapid growth, the shoot demand for N may exceed the N available 57 from the soil and/or BNF. Under these conditions, the addition of small amounts of readily available fertilizer N could result in increased dry matter accumulation. 58 Conclusions Under field conditions, the N nutrition of alfalfa involves differing contributions of soil N and BNF. The contribution that each N pool makes to plant N requirements is strongly influenced by the environment, particularly the efficiency of the legume-Rhizobium symbiosis, soil nitrate levels, and soil temperature. Because of the generally antagonistic effect of nitrate on BNF, as well as it's tendency to increase weed growth, it is not normally desirable to apply fertilizer N in legume establishment (Eardly et al., 1985). Most studies have shown that the yield of properly established, effectively nodulated alfalfa does not respond to N fertilizer. For the same reason, the application of manures or other N sources is not generally recommended as a desirable alfalfa production practice. However, studies with alfalfa (Peters and Kelling, 1989; Fishbeck and Phillips, 1981) and other legumes (Schomberg and Weaver, 1990) suggest that in low N soils, some yield response to starter N fertilization may be observed. When alfalfa is established in a low N envi- ronment and shoot demand for N exceeds the quantity of N available from soil N or BNF, an N deficit will develop (MacDuff et al., 1989). Our studies suggest that this N 59 deficit is exacerbated by low soil temperatures during the development of the symbiosis. Alfalfa dry matter yield and PBNF exhibited the greatest response to small amounts of preplant N (20-40 kg ha-1) when the day/night soil temperature was 18/12°C. An accurate prediction of the N-fertilizer requirements of a non-N2-fixing crop involves a consideration of the quantity and distribution of inorganic N in the soil, the demand of the crop for N, and the ability of the root system to extract inorganic N from soil (Greenwood, 1986). In legumes, the ability of the legume-Rhizobium symbiosis to supply N over time also must be considered. Some general guidelines for the use of preplant N in alfalfa may be derived from these experiments. The pre- treatment 2 M KC1 extractable nitrate concentration in the field experiments at Powell Butte in 1988, when a yield response to 20 kg ha-1 preplant N was observed, ranged from 13-20 mg kg-1. The average daily soil tem- perature at 10 cm for the two weeks after planting was 10°C. In growth chamber experiments, the greatest dry matter and PBNF response to fertilizer N was at a soil temperature of 18/12°C. level was about 6 mg kg-1. The average initial soil nitrate Statistical analysis of growth chamber studies indicated that temperature and temperature X N interaction effects are more important 60 than N effects. This may explain why a response to N in the field was observed even though the initial nitrate level was higher than in the soil used in the growth chamber study. Based on the field conditions in these experiments, 20-40 kg ha-1 preplant N would result in greater yield at first harvest only if the average daily soil temperature for at least two weeks after planting is less than 15°C and the soil nitrate level before planting is less than 16 mg kg-1. In any production practice, growers must consider the cost/benefit ratio in regard to production goals. To correctly assess the need for preplant N in alfalfa establishment, both soil available N and soil temperature at planting should be considered. To establish a repre- sentative profile of soil temperature flucuations, weather records of nearby weather stations have records of daily 10 cm (4") and 20 cm (8") soil temperature. If daily average soil temperatures are below 15°C for at least two weeks after normal planting time, it may be advisable to plant later in the season. If this is not feasible, and if both soil nitrate and soil temperature profiles indicate a potential N deficit, a small application of preplant N (20-40 kg ha-1) may provide an in- crease in first harvest yield. If there is an adequate population of Rhizobium meliloti present, this amount of 61 N should not result in reduced seasonal PBNF and thus BNF will eventually be able to supply most of the plant N needs. 62 References Allos, H.F., and W.V. Bartholomew. 1959. Replacement of symbiotic fixation by available nitrogen. Soil Sci. 87:61-66. Barber, S.A. 1984. Soil nutrient bioavailability. John Wiley and sons, New York. Barnes, D.K., B.P. Goplen, and J.E. Baylor. 1988. Highlights in the USA and Canada. p. 1-24. In A.A. Hanson (ed.) Alfalfa and alfalfa improvement. 2nd ed. Academic Press, New York. Barta, A.L. 1978. Effect of root temperature on dry matter distribution, carbohydrate accumulation and acetylene reduction in alfalfa and birdsfoot trefoil. Crop Sci. 18:637-640. Bremner, J.M., and C.S. Mulvaney. 1982. Nitrogen-total. p. 595-622. In A.L. Page (ed.) Methods of soil analysis. Part 2. ASA. Madison, WI. Date, R.A., and R.J. Roughley. 1986. Effects of root temperature on the growth and nitrogen fixation of Trifolium semipilosum and Trifolium repens. Exp. Agric. 22:133-147. Eardly, B.D., Hannaway, D.B., and P.J. Bottomley. 1985. Nitrogen nutrition and yield of seedling alfalfa as affected by ammonium nitrate fertilization. Agron. J. 77:57-62. Eardly, B.D., D.B. Hannaway, and P.J. Bottomley. 1988. Residual effects of Rhizobium and preplant N fertilizer on newly established alfalfa. J. Agron. & Crop Sci. 161:310-315. Fick, G.W., D.A. Holt, and D.G. Lugg. 1988. Environmental physiology and crop growth. p. 163-194. In A.A. Hanson (ed.) Alfalfa and alfalfa improvement. Academic press, New York. Fick, G.W., and S.C. Mueller. 1989. Alfalfa quality, maturity, and mean stage of development. Cornell Univ. Coop. Ext. Inf. Bull. 217. 63 Fishbeck, K.A., and D.A. Phillips. 1981. Combined nitrogen and vegetative regrowth of symbiotically-grown alfalfa. Agron. J. 73:975-978. Gates, R.P.G., and J.H. Silsbury. 1987. Effects of temperature on growth and nitrogen fixation by swards of subterranean clover. Ann. Bot. 59:461-469. Gordon, A.J., J.H. MacDuff, G.J.A. Ryle, and C.E. Powell. 1989. White clover N2-fixation in response to root temperature and nitrate. II. N-fixation, respiration and nitrate reductase activity. J. Exp. Bot. 40:527-534. Greenwood, D.J. 1986. Prediction of nitrogen fertilizer needs of arable crops. p. 1-61. In B. Tinker and A. Lauchli (ed.) Adv. P1. Nutr. Vol 2. Praeger Publ. New York. Henson, R.A., and G.H. Heichel. 1984. Dinitrogen fixation of soybean and alfalfa: comparison of the isotope dilution and difference methods. Field crops res. 9:333-346. Heichel, G.H., and C.P. Vance. 1979. Nitrate-N and rhizobium strain roles in alfalfa seedling nodulation and growth. Crop Sci. 19:512-518. Heichel, G.H., D.K. Barnes, and C.P. Vance. 1981. Nitrogen fixation of alfalfa in the seedling year. Crop Sci. 21:330-335. Heichel, G.H., D.K. Barnes, C.P. Vance, and C.C. Sheaffer. 1989. Dinitrogen fixation technologies for alfalfa improvement. J. Prod. Agric. 2:24-32. Hojjati, S.M., W.C. Templeton, Jr., and T.H. Taylor. 1978. Nitrogen fertilization in establishing forage legumes. Agron. J. 70:429-433. Jones, F.R., and W.B. Tisdale. 1921. Effect of soil temperature upon the development of nodules on the roots of certain legumes. J. Agr. Res. 22:17-31. Keeney, D.R., and D.W. Nelson. 1982. Nitrogen-inorganic forms. p. 643-698. In A.L. Page et al. (ed.) Methods of soil analysis. Part 2. 2nd ed. Agron. Monogr. 9. ASA, Madison, WI. 64 Lee, C., and D. Smith. 1972. Influence of nitrogen fertilizer on stands, yields of herbage and protein, and nitrogenous fractions of field grown alfalfa. Agron. J. 64:527-530. Mahon, J.D., and J.J. Child. 1979. Growth response of inoculated peas (Pisium sativum) to combined nitrogen. Can J. Bot. 57:1687-1693. MacDowall, F.D.H. 1982. Effects of root environment on the kinetics of 1st month growth and nodulation of alfalfa. Can. J. Bot. 60:888-896. MacDuff, J.H., A.J. Gordon, G.J.A. Ryle, and C.E. Powell. 1989. White clover N2-fixation in response to root temperature and nitrate. J. Exp. Bot. 40:517-526. Peters, E.J., and J.F. Stritzke. 1970. Herbicides and nitrogen fertilizer for the establishment of three varieties of spring-sown alfalfa. Agron. J. 62:259262. Peters, J.B, and K.A. Kelling. 1987. Ammonium sulfate as a concomitant N and S source for alfalfa. WI Fert., Aglime and Pest Mgt. Conf. 26:66-75. Peters, J.B., and K.A. Kelling. 1989. Interaction of pH and N on alfalfa establishment, yield and stand persistence. Proc. 13th WI Forage Council Symp. 13:114-122. SAS. 1990. SAS/STAT users guide, version 6, fourth edition, volume 1. SAS Institute, Cary, NC. Schomberg, H.H., and R.W. Weaver. 1990. Early growth and dinitrogen fixation by arrowleaf clover in response to starter nitrogen. Agron. J. 82:946-951. Sharratt, B.S., C.C. Sheaffer, and D.G. Baker. 1989. Base temperature for the application of the growingdegree-day model to field-grown alfalfa. Field Crops Res. 21:95-102. Simson, C.R., J.B. Peters, and K.A. Kelling. 1981. Influence of nitrogen fertilizer on alfalfa establishment. WI Fert., Aglime and Pest Mgt. Conf. 20:59-64. Smith, D. 1970. Influence of temperature on the yield and chemical composition of five forage legume species. Agron. J. 62:520-523. 65 Streeter, J.G., 1988. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 7:1-23. Vance, C.P., G.H. Heichel, and D.A. Phillips. 1988. Nodulation and symbiotic dinitrogen fixation. p. 229257. In A.A. Hanson (ed.) Alfalfa and alfalfa improvement. Academic Press, New York. Ward, C.Y., and R.E. Blaser. 1961. Effect of nitrogen fertilizer on emergence and seedling growth of forage plants and subsequent production. Agron. J. 53:115-120. Wery, J., 0. Turc, and L. Salsac. 1986. Relationship between growth, nitrogen fixation and assimilation in a legume (Medicago sativa L.). Plant and Soil 96:1729. 66 IV. BIBLIOGRAPHY Allos, H.F., and W.V. Bartholomew. 1959. Replacement of symbiotic fixation by available nitrogen. Soil Sci. 87:61-66. Atkins, C.A. 1986. The legume/Rhizobium symbiosis: limitations to maximizing nitrogen fixation. Outlook on Agriculture 15:128-134. Barber, S.A. 1984. Soil nutrient bioavailability. John Wiley and sons, New York. Barnes, D.K., B.P. Goplen, and J.E. Baylor. 1988a. Highlights in the USA and Canada. p. 1-24. In A.A. Hanson (ed.) Alfalfa and Alfalfa Improvement. Academic Press, New York. Barnes, D.K., C.P. Vance, G.H. Heichel, M.A. Peterson, and W.R. Ellis. 1988b. Registration of a non-nodulation and three ineffective nodulation alfalfa germplasms. Crop Sci. 28:721-722. Barnes, D.K., G.H. Heichel, C.P. Vance, and W.R. Ellis. 1984. A multiple trait breeding program for improving the symbiosis for N2 fixation between Medicago sativa L. and Rhizobium meliloti. Plant Soil 82:303-314. Barrios, S., N. Raggio, and M. Raggio. 1963. Effect of temperature of infection of isolated bean roots by rhizobia. Plant Physiol. 46:171-175. Barta, A.L. 1978. Effect of root temperature on dry matter distribution, carbohydrate accumulation and acetylene reduction in alfalfa and birdsfoot trefoil. Crop Sci. 18:637-640. Becana, M., and J.I. Sprent. 1987. Nitrogen fixation and nitrate reduction in the root nodules of legumes. Physiol. Plant. 70:757-765. Bremner, J.M., and C.S. Mulvaney. 1982. Nitrogen-total. p. 595-622. In A.L. Page (ed.) Methods of soil analysis. Part 2. ASA. Madison, WI. Burton, J.C. 1972. Nodulation and nitrogen fixation in alfalfa. p. 229-246. In C.H. Hansen (ed.) Alfalfa science and technology. Agronomy 15:229-246. 67 Burton, J.C., and L.W. Erdman. 1940. A division of the alfalfa cross-inoculation group correlating efficiency in nitrogen fixation with source of Rhizobium meliloti. Agron. J. 32:439-450. Chalk, P.M. 1985. Estimation of N2 fixation by isotcWe dilution: an appraisal of techniques involving N enrichment and their application. Soil Biol. Biochem. 17:389-410. Collins, M., and S.H. Duke. 1981. Influence of potassiumfertilization rate and form on photosynthesis and N2 fixation of alfalfa. Crop Sci. 21:481-484. Criswell, J.B. R.W.F. Hardy, and V.D. Havelka. 1976. Nitrogen fixation in soybeans: measurement techniques and examples of application. p. 108-124. In L.D. Hill (ed.) World soybean research. Interstate Printers and Publishers, Danville, IL. Dart, P.J., J.M. Day, R. Islam, and J. Dobereiner. 1975. Symbiosis in tropical grain legumes -- some effects of temperature and the composition of the rooting medium. p. 361-384. In Nutman, P.S. (ed.) Symbiotic nitrogen fixation in plants. Cambridge University Press. Das, G. 1982. A stimulatory effect of low nitrate levels on nodulation, leghemoglobin content, nitrogenase activity, and growth of mung-bean plants (Vigna radiata). Can. J. Bot. 60:1907-1912. Date, R.A., and R.J. Roughley. 1986. Effects of root temperature on the growth and nitrogen fixation of Trifolium semipilosum and Trifolium repens. Exper. Agric. 22:133-147. Day, J.M., and P.J. Dart. 1971. Effect of temperature and combined nitrogen on nodule structure. p. 84-85. Rothamsted Exp. Sta. Rep. 1970, Pt. 1. Dazzo, F.B., and W.J. Brill. 1978. Regulation by fixed nitrogen of host-symbiont recognition in the Rhizobium-clover symbiosis. Plant Physiol. 62:18-21. Dreyfus, B.L., H.G. Diem, and Y.R. Dommergues. 1988. Future directions for biological nitrogen fixation research. Plant and Soil 108:191-199. 68 Duke, S.H., and D.C. Doehlert. 1981. Root respiration, nodulation, and enzyme activities in alfalfa during cold acclimation. Crop Sci. 21:489-495. Eaglesham, A.R.J., S. Hassouna, and R. Seegers. 1983. Fertilizer-N effects on N2 fixation by cowpea and soybean. Agron. J. 75:61-66. Eardly, B.D., Hannaway, D.B., and P.J. Bottomley. 1985. Nitrogen nutrition and yield of seedling alfalfa as affected by ammonium nitrate fertilization. Agron. J. 77:57-62. Eardly, B.D., Hannaway, D.B., and P.J. Bottomley. 1988. Residual effects of Rhizobium and preplant N fertilizer on newly established alfalfa. J. Agron. Crop Sci. 161:310-315. Ek-Jander, J., and G. Fahraeus. 1971. Adaptation of Rhizobium to subarctic environment in Scandinavia. p. 129-138. In T.A. Lie and E.G. Mulder (ed.) Biological nitrogen fixation in natural and agricultural habitats. Proc. Tech. Meet. on Biol. N Fix. of the Int. Biol. Prog., Prague and Wageningen, 1970. Plant Soil Spec. Vol. FAO. 1984. Legume inoculants and their use. FAO Fert. and P1. Nutr. Serv. FAO, Rome. Fick, G.W., and S.C. Mueller. 1989. Alfalfa quality, maturity, and mean stage of development. Cornell Univ. Coop. Ext. Bull. 217. Fick, G.W., D.A. Holt, and D.G. Lugg. 1988. Environmental physiology and crop growth. p. 163-194. In A.A. Hanson (ed.) Alfalfa and alfalfa improvement. Academic press, New York. Fishbeck, K.A., and D.A. Phillips. 1981. Combined nitrogen and vegetative regrowth of symbiotically-grown alfalfa. Agron. J. 73:975-978. Gates, R.P.G., and J.H. Silsbury. 1987. Effects of temperature on growth and nitrogen fixation by swards of subterranean clover. Ann. Bot. 59:461-469. Gibson, A.H. 1974. Consideration of the growing legume as a symbiotic association. Proc. Indian Nat. Sci. Acad. 40(B6):741-767. 69 Gibson, A.H. 1962. Genetic variation in the effectiveness of nodulation of lucerne varieties. Aust. J. Agric. Res. 13:388-399. Giller, K.E. 1987. Use and abuse of acetylene reduction assay for measurement of associated nitrogen fixation. Soil Biol. Biochem. 19:783-784. Gist, G.R., and G.O. Mott. 1957. Some effects of light intensity, temperature, and soil moisture on the growth of alfalfa, red clover, and birdsfoot trefoil seedlings. Agron. J. 49:33-36. Gordon, A.J., J.H. MacDuff, G.J.A. Ryle, and C.E. Powell. 1989. White clover N2-fixation in response to root temperature and nitrate. II. N2-fixation, respiration and nitrate reductase activity. J. Exp. Bot. 40:527534. Greenwood, D.J. 1986. Prediction of nitrogen fertilizer needs of arable crops. p. 1-61. In B. Tinker and A. Lauchli (ed.) Adv. Pl. Nutr. Vol 2. Praeger Publ. New York. Harding, S.C., and J.E. Sheehy. 1980. Influence of shoot and root temperature on leaf growth, photosynthesis and nitrogen fixation of lucerne. Ann. Bot. 45:229233. Hauch, R.D., and R.W. Weaver (ed.). 1986. Field measurement of dinitrogen fixation and denitrification. SSSA Spec. Publ. 18, Madison, WI. Haystead, A., J. King, and W.I.C. Lamb. 1980. Photosynthesis, respiration and nitrogen fixation in white clover. Grass Forage Sci. 34:125-130. Heichel, G.H., and C.P. Vance. 1979. Nitrate-N and rhizobium strain roles in alfalfa seedling nodulation and growth. Crop Sci. 19:512-518. Heichel, G.H., D.K. Barnes, and C.P. Vance. 1981. Nitrogen fixation of alfalfa in the seedling year. Crop Sci. 21:330-335. 70 Heichel, G.H., C.P. Vance, and D.K. Barnes. 1983. Symbiotic nitrogen fixation of alfalfa, birdsfoot trefoil, and red clover. p. 336-338. In J.A. Smith and V.W. Hays (ed.) Proc. 14th Int. Grassi. Congr., Lexington, Ky. 15-24 June 1981. Westview Press, Boulder, CO. Heichel, G.H., D.K. Barnes, C.P. Vance, and K.I. Henjum. 1984. N2 fixation, N and dry matter partitioning during a 4-year alfalfa stand. Crop Sci. 24:811-815. Heichel, G.H., D.K. Barnes, C.P. Vance, and C.C. Sheaffer. 1989. Dinitrogen fixation technologies for alfalfa improvement. J. Prod. Agric. 2:24-32. Henson, R.A., and G.H. Heichel. 1984. Dinitrogen fixation of soybean and alfalfa: comparison of the isotope dilution and difference methods. Field Crops Res. 9:333-346. Hesterman, 0.B., and L.R. Teuber. 1981. Effect of photoperiod and irradiance on fall dormancy of alfalfa. Agron. Abstr. Amer. Soc. of Agron., Madison, WI. p. 87. Hojjati, S.M., W.C. Templeton, Jr., and T.H. Taylor. 1978. Nitrogen fertilization in establishing forage legumes. Agron. J. 70:429-433. Jensen, E.H., M.A. Massengale, and D.O. Chilcote. 1967. Environmental effects on growth and quality of alfalfa. Nevada Agric. Exp. Stn. Bull. T9. Jones, F.R., and W.B. Tisdale. 1921. Effect of soil temperature upon the development of nodules on the roots of certain legumes. J. Agr. Res. 22:17-31. Keeney, D.R., and D.W. Nelson. 1982. Nitrogen-inorganic forms. p. 643-698. In A.L. Page (ed.) Methods of soil analysis. Part 2. 2nd ed. Agron. Monogr. 9. ASA, Madison, WI. Kunelius, H.T. 1974. Effects of weed control and N fertilization at establishment on the growth and nodulation of alfalfa. Agron. J. 66:806-809. Lee, C., and D. Smith. 1972. Influence of nitrogen fertilizer on stands, yields of herbage and protein, and nitrogenous fractions of field grown alfalfa. Agron. J. 64:527-530. 71 Lie, T.A. 1971. Symbiotic nitrogen fixation under stress conditions. p. 117-128. In T.A. Lie and E.G. Mulder (ed.) Biological nitrogen fixation in natural and agricultural habitats. Proc. Tech. Meet. on Biol. Nitr. Fix. of the Int. Biol. Prog., Prague and Wageningen, 1970. Plant Soil Spec. Vol. Lie, T. 1974. Environmental effects on nodulation and symbiotic nitrogen fixation. p. 555-582. In A. Quispel (ed.) The biology of nitrogen fixation. Frontiers of Biol. Vol 33. North-Holland Pub. Co., Oxford. MacDowall, F.D.H. 1982. Effects of root environment on the kinetics of 1st month growth and nodulation of alfalfa. Can. J. Bot. 60:888-896. MacDuff, J.H., A.J. Gordon, G.J.A. Ryle, and C.E. Powell. 1989. White clover N2-fixation in response to root temperature and nitrate. J. Exp. Bot. 40:517-526. Mague, T.H., and R.H. Burris. 1972. Reduction of acetylene and nitrogen by field-grown soybean plants. New Phytol. 71:275-286. Mahon, J.D., and J.J. Child. 1979. Growth response of inoculated peas (Pisium sativum) to combined nitrogen. Can J. Bot. 57:1687-1693. McAuliffe, C., D.S. Chamblee, H. Uribi-Arango, and W.W. Woodhouse, Jr. 1958. Influence of inorganic nitrogen on nitrogen fixation by legumes as revealed by N. Agron. J. 50:334-337. McElgunn, J.D. 1973. Germination response of forage legumes to constant and alternating temperatures. Can. J. Plant Sci. 53:797-800. Mederski, H.J., and J.G. Streeter. 1977. Continuous automated acetylene reduction assays using intact plants. Plant Physiol. 59:1076-1081. Minchin, F.R., J.E. Sheehy, J.F. Witty, J.A. Stuart, and R.E. Cooper. 1982. Respiration and symbiotic nitrogen fixation in white clover. p. 37-38. In Donaldson, W.A.D. and K.M. Down (ed.) Annual Rep. 1981, Grassl. Res. Inst., Hurley. 72 Minchin, F.R., J.F. Witty, J.F. Sheehy. 1983. A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. Journ. of Exp. Bot. 34:641-649. Minchin, F.R., M.I. Minguez, J.E. Sheehy, J.F. Witty, and L. Skot. 1986. Further errors in the acetylene reduction assay: effects of plant disturbance. J. Exp. Bot. 37:1581-1591. Munns, D.N. 1977. Mineral nutrition and the legume symbiosis. p. 353-391. In R.W.F. Hardy and A.H. Gibson (ed.) A treatise on dinitrogen fixation, IV: Agronomy and Ecology. John Wiley and Sons, New York. Oregon Department of Agriculture. 1990. Oregon agriculture & fisheries statistics. Oregon Dept. of Agric. Salem, OR. Pearson, C.J., and L.A. Hunt. 1972. Effects of temperature on primary growth and regrowths of alfalfa. Can. J. Plant Sci. 52:1017-1027. Peters, E.J. 1964. Pre-emergence, preplanting and postemergence herbicides for alfalfa and birdsfoot trefoil. Agron. J. 56:415-419. Peters, J.B, and K.A. Kelling. 1987. Ammonium sulfate as a concomitant N and S source for alfalfa. WI Fert., Aglime and Pest Mgt. Conf. 26:66-75. Peters, J.B., and K.A. Kelling. 1989. Interaction of pH and N on alfalfa establishment, yield and stand persistence. Proc. 13th WI Forage Council Symp. 13:114-122. Peters, E.J., and J.F. Stritzke. 1970. Herbicides and nitrogen fertilizer for the establishment of three varieties of spring-sown alfalfa. Agron. J. 62:259262. Petersen, R.G. 1985. Design and analysis of experiments. Marcel Dekker, New York. Rawsthorne, S., P. Hadley, R.J. Summerfield, and E.H. Roberts. 1985. Effects of supplemental nitrate and thermal regime on the nitrogen nutrition of chick pea (Cicer arietinum L.). II. Symbiotic development and nitrogen assimilation. Plant Soil 83:279-293. 73 Richardson, D.A., D.C. Jordan, and E.H. Garrard. 1957. The influence of combined nitrogen on nodulation and nitrogen fixation by Rhizobium meliloti Dangeard. Can. J. Plant Sci. 37:205-214. Roughley, R.J. 1970. The influence of root temperature, Rhizobium strain and host selection on the structure and nitrogen-fixing efficiency of the root nodules of Trifolium subterraneum. Ann. Bot. 34:631-46. Roughley, R.J., and R.A. Date. 1986. The effect of strain of Rhizobium and of temperature on nodulation and early growth of Trifolium semipilosum. Exp. Agric. Cambridge 22:123-131. Ruschel, A.P., P.B. Vose, R.L. Victoria, and E. Salati. 1979. Comparison of isotope techniques and non-nodulating isolines to study the effect of ammonium fertilization on dinitrogen fixation in soybean, Glycine max. Plant Soil 53:513-525. SAS. 1990. SAS/STAT users guide, version 6, fourth edition, volume 1. SAS Institute, Cary, NC. Schomberg, H.H., and R.W. Weaver. 1990. Early growth and dinitrogen fixation by arrowleaf clover in response to starter nitrogen. Agron. J. 82:946-951. Sharratt, B.S., C.C. Sheaffer, and D.G. Baker. 1989. Base temperature for the application of the growing-degree-day model to field-grown alfalfa. Field crops res. 21:95-102. Sibma, L., and J.H.J. Spiertz. 1986. Dry matter production and nitrogen utilization in cropping systems with grass, lucerne, and maize. 1. Comparison of crop characteristics, growth, and production. Neth. J. Agric. Sci. 34:25-35. Silvester, W.B., R. Parsons, F.R. Minchin, and J.F. Witty. 1989. Simple apparatus for growth of nodulated plants and for continuous nitrogenase assay under defined gas phase. p. 55-66. In J.G. Torrey and L.J. Winship (ed.) Applications of continuous and steady state methods to root biology. Kluwer Academic Publ., Dordrectht. Simson, C.R., J.B. Peters, and K.A. Kelling. 1981. Influence of nitrogen fertilizer on alfalfa establishment. WI Fert., Aglime and Pest Mgt. Conf. 20:59-64. 74 Smith, D. 1970. Influence of temperature on the yield and chemical composition of five forage legume species. Agron. J. 62:520-523. Smith, S.C., D.F. Bezdicek, R.F. Turco, and H.H. Cheng. 1987. Seasonal N 215fixation by cool-season pulses based on several N methods. Plant and Soil 97:3-13. Sprent, J.I., and P. Sprent. 1990. Nitrogen fixing organisms. Pure and applied aspects. Chapman and Hall, London. Sprent, J.I., and J.R. Raven. 1985. Evolution of nitrogen-fixing symbioses. Proc. Roy. Soc. Edinb. 85B:215237. Stone, J.E., D.B. Marx, and A.K. Dobrenz. 1979. Interaction of sodium chloride and temperature on germination of two alfalfa cultivars. Agron. J. 71:425-427. Streeter, J.G., 1988. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 7:1-23. Tadmor, N.H., L. Shannon, and M. Evenari. 1971. Runoff farming in the desert. V. Persistence and yields of annual range species. Agron. J. 63:91-95. Tan, G.Y. 1981. Genetic variation for acetylene reduction rate and other characters in alfalfa. Crop Sci. 21:485-488. Teuber, L.R., R.P. Levin, T.C. Sweeney, and D.A. Phillips. 1984. Selection for N concentration and forage yield in alfalfa. Crop Sci. 24:553-558. Teuber, L.R., and D.A. Phillips. 1988. Influences of selection method and nitrogen environment on breeding alfalfa for increased forage yield and quality. Crop Sci. 28:599-604. Townsend, C.E., and W.J. McGinnies. 1972. Temperature requirements for seed germination of several forage legumes. Agron. J. 64:809-812. Triplett, G.B., Jr., and M.B. Tesar. 1960. Effects of compaction, depth of planting, and soil moisture tension on seedling emergence of alfalfa. Agron. J. 52:681-684. 75 Truchet, G.L., and F.B. Dazzo. 1982. Morphogenesis of lucerne root nodules incited by Rhizobium meliloti in the presence of combined nitrogen. Planta 154:352360. Vance, C.P., G.H. Heichel, and D.A. Phillips. 1988. Nodulation and symbiotic dinitrogen fixation. p. 229257. In A.A. Hanson (ed.) Alfalfa and alfalfa improvement. Academic Press, New York. Vincent, J.M. 1977. Rhizobium: general microbiology. p. 277-350. In R.W.F. Hardy and W.S. Silvac (ed.) A treatise on dinitrogen fixation. John Wiley & Sons, New York. Ward, C.Y., and R.E. Blaser. 1961. Effect of nitrogen fertilizer on emergence and seedling growth of forage plants and subsequent production. Agron. J. 53:115120. Washko, J.B., and J.W. Price. 1970. Intensive management of alfalfa for forage production. p. 628-632. Proc. XI Int. Grassi. Cong., Queensland, Australia. Waughman, E.J. 1977. The effect of temperature on nitrogenase activity. J. Exp. Bot. 28:949-960. Wery, J., 0. Turc, and L. Salsac. 1986. Relationship between growth, nitrogen fixation and assimilation in a legume (Medicago sativa L.). Plant and Soil 96:17-29. West, C.P., and W.F. Wedin. 1985. Dinitrogen fixation in alfalfa-orchardgrass pastures. Agron. J. 77:89-94. Winteringham, F.P.W. 1984. Soil and fertilizer nitrogen. Technical Report, IAEA series No. 244. Vienna. Witty, J.F1.5 1983. Estimating N2-fixation in the field using N-labelled fertilizer: Some problems and solutions. Soil Biol. Biochem. 15:631-639. Wivstad, M., A.M. Martensson, and H.D. Ljunggren. 1987. Field measurement of symbiotic nitrogen fixation in an established lucerne ley using N and an acetylene reduction method. Plant and Soil 97:93-104. V. APPENDIX Table 1. Analysis of variance of dry matter yield for 1987 and 1988 field experiments at Powell Butte, OR. Source DF Sum of squares Total 38 551936040.38 Year 1 Replicate N Level Error Mean square F value P > F 6433662.57 6433662.57 0.53 0.4728 3 1609333.51 536444.50 0.04 0.9875 4 178729622.07 44682405.52 3.67 0.0151 30 365163422.23 Table 2. Shoot and root dry matter accumulation of alfalfa grown at a day/night soil temperature regime of 18/12°C and five levels of preplant N in growth chamber experiments. Preplant N Shoots Roots Days after planting Days after planting 47 123 219 kg ha-1 252 47 123 219 252 g plant.1 0 0.1 0.5 1.8 1.6 0.1 0.5 1.9 3.4 10 0.1 0.4 1.1 1.2 0.1 0.4 1.2 2.1 20 0.1 0.4 1.4 1.8 0.1 0.4 1.8 2.7 40 0.1 0.4 1.1 2.7 0.1 0.4 1.5 3.4 80 0.1 0.4 2.0 2.5 0.1 0.4 1.9 4.3 Table 3. Shoot and root dry matter accumulation of alfalfa grown at a day/night soil temperature regime of 24/16°C and five levels of preplant N in growth chamber experiments. Preplant N Shoots Roots Days after planting Days after planting 44 76 95 kg ha-1 134 44 76 95 134 g plant-1 0 0.1 0.2 0.3 1.4 0.1 0.2 0.3 1.3 10 0.1 0.3 0.4 1.6 0.1 0.3 0.4 1.4 20 0.1 0.2 0.4 1.4 0.1 0.3 0.4 1.3 40 0.1 0.2 0.4 2.0 0.1 0.2 0.4 1.6 80 0.1 0.3 0.5 1.5 0.1 0.3 0.5 1.4 Table 4. Shoot and root dry matter accumulation of alfalfa grown at a day/night soil temperature regime of 27/21°C and five levels of preplant N in growth chamber experiments. Preplant N Shoots Roots Days after planting Days after planting 73 85 107 kg ha-1 145 73 85 107 145 g plant-1 0 0.1 0.2 0.3 0.8 0.1 0.2 0.2 0.6 10 0.1 0.2 0.3 0.8 0.1 0.1 0.2 0.7 20 0.1 0.2 0.3 0.8 0.1 0.2 0.2 0.7 40 0.1 0.2 0.4 0.7 0.1 0.2 0.3 0.7 80 0.1 0.2 0.3 0.8 0.1 0.2 0.2 0.6 Table 5. Shoot and root N concentration of alfalfa grown at a day/night soil temperature regime of 18/12°C and five levels of preplant N in growth chamber experiments. Preplant N Shoots Roots Days after planting Days after planting 47 123 219 kg ha-1 252 g N 47 123 219 252 kg-1 0 8.8 22.0 19.8 20.5 29.3 29.8 25.5 22.8 10 8.8 21.5 19.2 20.0 29.1 30.0 27.5 25.8 20 6.2 20.8 18.8 20.0 29.0 28.8 24.5 25.2 40 4.6 21.2 24.2 20.5 30.0 28.8 28.0 24.8 80 2.6 20.2 23.0 22.0 29.2 29.2 26.5 25.0 Table 6. Shoot and root N concentration of alfalfa grown at a day/night soil temperature regime of 24/16°C and five levels of preplant N in growth chamber experiments. Preplant N Shoots Roots Days after planting Days after planting 44 76 95 134 g N kg ha-1 44 76 95 134 kg-1 0 29.7 30.2 22.2 25.3 17.9 19.6 26.0 20.0 10 31.9 29.6 23.5 25.6 20.0 19.0 23.2 21.6 20 30.0 29.8 22.8 24.2 18.5 17.1 15.2 22.6 40 29.3 29.7 24.5 23.9 18.0 18.1 25.5 21.5 80 29.0 29.2 22.8 23.1 17.9 19.2 24.5 20.6 Table 7. Shoot and root N concentration of alfalfa grown at a day/night soil temperature regime of 27/21°C and five levels of preplant N in growth chamber experiments. Preplant N Shoots Roots Days after planting Days after planting 73 85 107 kg ha-1 145 g N 73 85 107 145 kg-1 0 20.6 19.1 22.4 21.9 32.2 28.6 31.8 26.0 10 20.8 20.9 21.3 21.3 31.7 31.5 28.6 28.9 20 20.3 21.6 21.1 21.7 32.7 29.6 31.3 29.7 40 20.4 19.0 21.4 21.8 32.0 27.2 29.1 27.9 80 19.2 19.0 20.4 22.6 31.5 29.4 31.4 29.2 Table 8. Shoot and root N content of alfalfa shoots grown at a day/night soil temperature regime of 18/12°C and five levels of preplant N in growth chamber experiments. Preplant N 47 Shoots Roots Days after planting Days after planting 123 219 kg ha-1 252 47 123 219 252 g N plant-1 0 0.1 1.0 1.0 3.2 0.1 1.4 1.2 5.9 10 0.1 0.8 1.2 2.5 0.1 1.1 1.7 5.4 20 0.1 0.7 1.1 3.5 0.1 1.0 1.4 6.8 40 0.1 0.8 1.6 5.7 0.1 1.2 2.0 8.5 80 0.1 0.7 3.3 5.5 0.1 1.0 3.8 10.9 Table 9. Shoot and root N content of alfalfa grown at a day/night soil temperature regime of 24/16°C and five levels of preplant N in growth chamber experiments. Preplant N 44 Shoots Roots Days after planting Days after planting 76 95 kg ha-1 134 44 76 95 134 g N plant-1 0 0.2 0.7 0.7 3.5 0.1 0.5 0.8 3.5 10 0.2 0.8 1.0 4.2 0.1 0.5 0.8 4.2 20 0.3 0.7 0.8 3.4 0.1 0.4 0.5 3.4 40 0.4 0.7 1.1 4.5 0.1 0.5 1.0 4.5 80 0.3 0.8 1.1 3.4 0.1 0.5 1.2 3.4 Table 10. Shoot and root N content of alfalfa grown at a day/night soil temperature regime of 27/21°C and five levels of preplant N in growth chamber experiments. Preplant N Shoots Roots Days after planting Days after planting 73 85 107 kg ha-1 145 73 85 107 145 g N plant-1 0 0.3 0.4 0.7 1.7 0.4 0.4 0.8 1.6 10 0.2 0.4 0.7 1.8 0.2 0.4 0.6 1.9 20 0.2 0.4 0.7 1.9 0.4 0.4 0.7 2.0 40 0.3 0.4 0.8 1.6 0.3 0.5 0.8 1.9 80 0.2 0.4 0.6 1.8 0.3 0.4 0.8 1.8