makeup water are to be determined. 2
advertisement
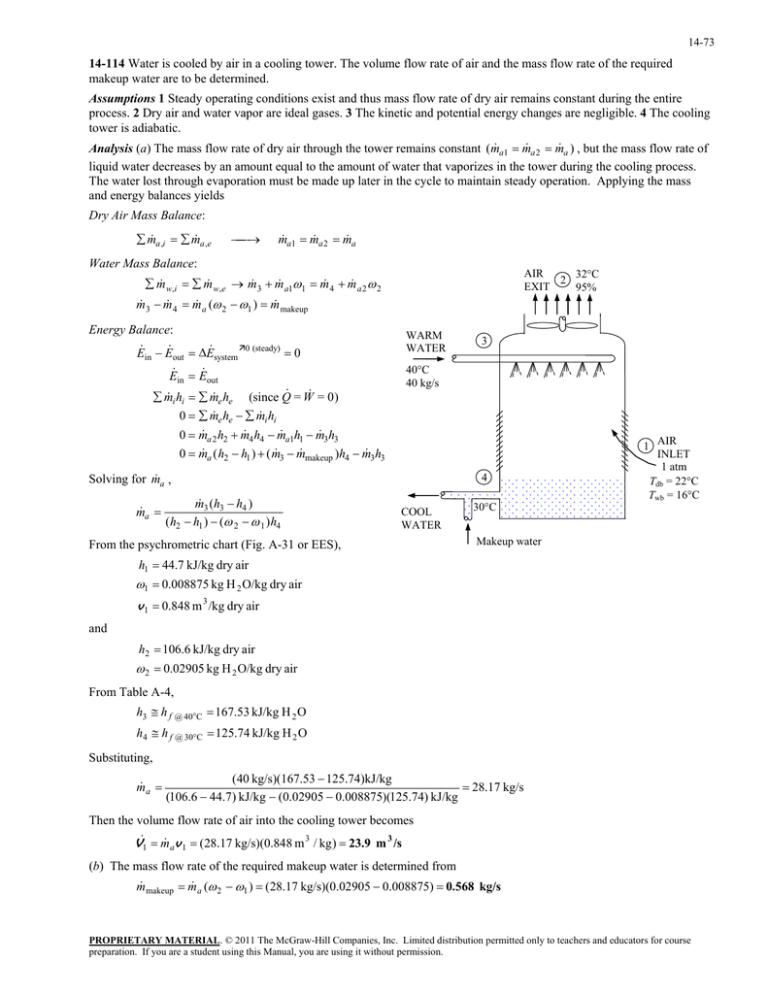
14-73 14-114 Water is cooled by air in a cooling tower. The volume flow rate of air and the mass flow rate of the required makeup water are to be determined. Assumptions 1 Steady operating conditions exist and thus mass flow rate of dry air remains constant during the entire process. 2 Dry air and water vapor are ideal gases. 3 The kinetic and potential energy changes are negligible. 4 The cooling tower is adiabatic. Analysis (a) The mass flow rate of dry air through the tower remains constant (m a1 m a 2 m a ) , but the mass flow rate of liquid water decreases by an amount equal to the amount of water that vaporizes in the tower during the cooling process. The water lost through evaporation must be made up later in the cycle to maintain steady operation. Applying the mass and energy balances yields Dry Air Mass Balance: m a ,i m a ,e m a1 m a 2 m a Water Mass Balance: AIR 32C 2 EXIT 95% m w,i m w,e m 3 m a11 m 4 m a 2 2 m 3 m 4 m a ( 2 1 ) m makeup Energy Balance: WARM WATER E in E out E system 0 (steady) 0 40C 40 kg/s E in E out i hi m e he (since Q = W = 0) m e he m i hi 0 m a 2 h2 m 4 h4 m a1h1 m 3h3 0m a ( h2 h1 ) ( m 3 m makeup )h4 m 3h3 0m Solving for m a , m a m 3 (h3 h4 ) (h2 h1 ) ( 2 1 )h4 3 4 COOL WATER From the psychrometric chart (Fig. A-31 or EES), 1 AIR INLET 1 atm Tdb = 22C Twb = 16C 30C Makeup water h1 44.7 kJ/kg dry air 1 0.008875 kg H 2 O/kg dry air v 1 0.848 m 3 /kg dry air and h2 106.6 kJ/kg dry air 2 0.02905 kg H 2 O/kg dry air From Table A-4, h3 h f @ 40C 167.53 kJ/kg H 2 O h4 h f @ 30C 125.74 kJ/kg H 2 O Substituting, m a (40 kg/s)(167.53 125.74)kJ/kg 28.17 kg/s (106.6 44.7) kJ/kg (0.02905 0.008875)(125.74) kJ/kg Then the volume flow rate of air into the cooling tower becomes V1 m av 1 (28.17 kg/s)(0.848 m 3 / kg ) 23.9 m 3 /s (b) The mass flow rate of the required makeup water is determined from m makeup m a ( 2 1 ) (28.17 kg/s)(0.02905 0.008875) 0.568 kg/s PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.