rises to a specified value. The total work and heat... , and N 13-96
advertisement
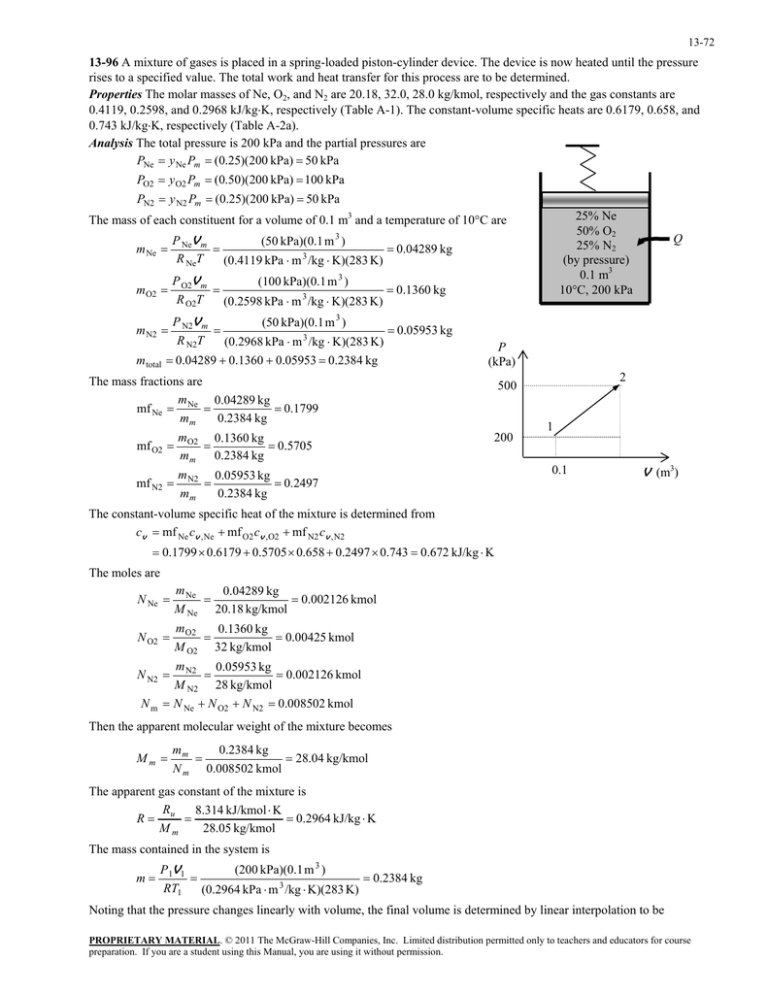
13-72 13-96 A mixture of gases is placed in a spring-loaded piston-cylinder device. The device is now heated until the pressure rises to a specified value. The total work and heat transfer for this process are to be determined. Properties The molar masses of Ne, O2, and N2 are 20.18, 32.0, 28.0 kg/kmol, respectively and the gas constants are 0.4119, 0.2598, and 0.2968 kJ/kgK, respectively (Table A-1). The constant-volume specific heats are 0.6179, 0.658, and 0.743 kJ/kgK, respectively (Table A-2a). Analysis The total pressure is 200 kPa and the partial pressures are PNe y Ne Pm (0.25)(200 kPa) 50 kPa PO2 y O2 Pm (0.50)(200 kPa) 100 kPa PN2 y N2 Pm (0.25)(200 kPa) 50 kPa 25% Ne 50% O2 25% N2 (by pressure) 0.1 m3 10C, 200 kPa The mass of each constituent for a volume of 0.1 m3 and a temperature of 10C are m Ne P NeV m (50 kPa)(0.1 m 3 ) 0.04289 kg R NeT (0.4119 kPa m 3 /kg K)(283 K) mO2 P O2V m (100 kPa)(0.1 m 3 ) 0.1360 kg R O2T (0.2598 kPa m 3 /kg K)(283 K) P N2V m (50 kPa)(0.1 m 3 ) 0.05953 kg R N2T (0.2968 kPa m 3 /kg K)(283 K) 0.04289 0.1360 0.05953 0.2384 kg Q m N2 m total P (kPa) The mass fractions are m 0.04289 kg 0.1799 mf Ne Ne mm 0.2384 kg mf O2 m 0.1360 kg O2 0.5705 m m 0.2384 kg mf N2 m 0.05953 kg N2 0.2497 mm 0.2384 kg 2 500 200 1 0.1 V (m3) The constant-volume specific heat of the mixture is determined from cv mf Ne cv , Ne mf O2 cv ,O2 mf N2 cv , N2 0.1799 0.6179 0.5705 0.658 0.2497 0.743 0.672 kJ/kg K The moles are N Ne m Ne 0.04289 kg 0.002126 kmol M Ne 20.18 kg/kmol N O2 m O2 0.1360 kg 0.00425 kmol M O2 32 kg/kmol N N2 m N2 0.05953 kg 0.002126 kmol M N2 28 kg/kmol N m N Ne N O2 N N2 0.008502 kmol Then the apparent molecular weight of the mixture becomes Mm mm 0.2384 kg 28.04 kg/kmol N m 0.008502 kmol The apparent gas constant of the mixture is R 8.314 kJ/kmol K R u 0.2964 kJ/kg K Mm 28.05 kg/kmol The mass contained in the system is m P 1V1 (200 kPa)(0.1 m 3 ) 0.2384 kg RT1 (0.2964 kPa m 3 /kg K)(283 K) Noting that the pressure changes linearly with volume, the final volume is determined by linear interpolation to be PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission. 13-73 500 200 V 2 0.1 V 2 0.4375 m 3 1000 200 1.0 0.1 The final temperature is T2 P 2V 2 (500 kPa)(0.4375 m 3 ) 3096 K mR (0.2384 kg)(0.2964 kPa m 3 /kg K) The work done during this process is P P (500 200) kPa (0.4375 0.1) m 3 118 kJ Wout 1 2 (V 2 V1 ) 2 2 An energy balance on the system gives Qin Wout mcv (T2 T1 ) 118 (0.2384 kg)(0.672 kJ/kg K )(3096 283) K 569 kJ PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.






