8-62 exit are to be determined.
advertisement
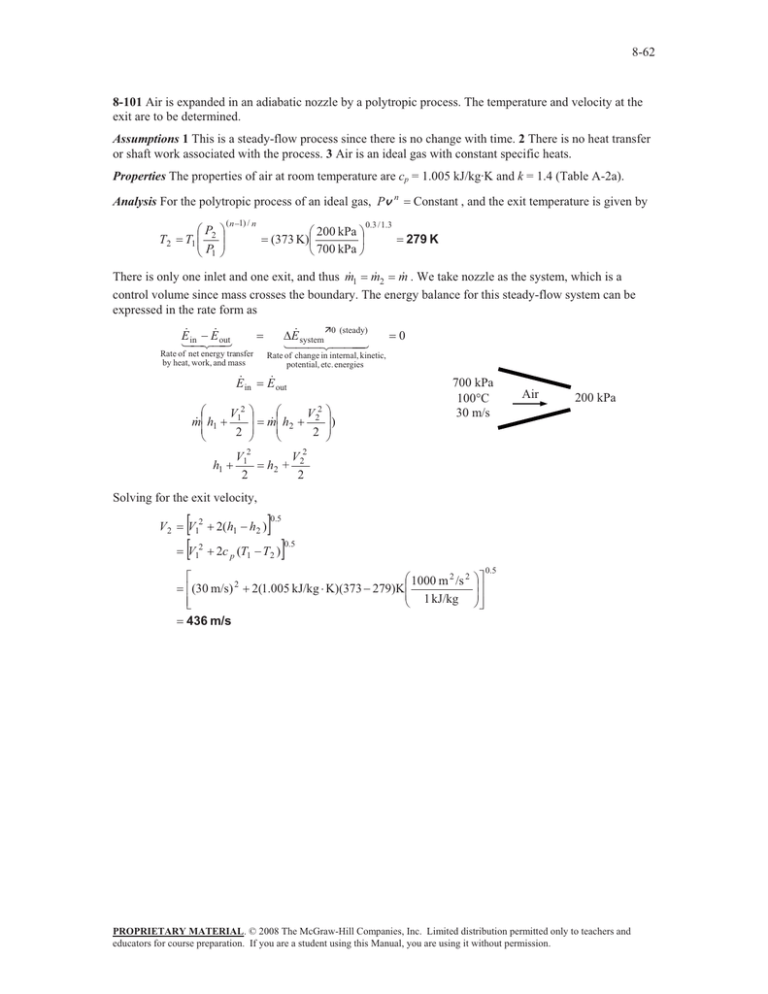
8-62 8-101 Air is expanded in an adiabatic nozzle by a polytropic process. The temperature and velocity at the exit are to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 There is no heat transfer or shaft work associated with the process. 3 Air is an ideal gas with constant specific heats. Properties The properties of air at room temperature are cp = 1.005 kJ/kg·K and k = 1.4 (Table A-2a). Analysis For the polytropic process of an ideal gas, Pv n T2 §P T1 ¨¨ 2 © P1 · ¸¸ ¹ ( n 1) / n § 200 kPa · (373 K)¨ ¸ © 700 kPa ¹ Constant , and the exit temperature is given by 0.3 / 1.3 279 K There is only one inlet and one exit, and thus m 1 m 2 m . We take nozzle as the system, which is a control volume since mass crosses the boundary. The energy balance for this steady-flow system can be expressed in the rate form as E E in out Rate of net energy transfer by heat, work, and mass E in § V2 m ¨ h1 1 ¨ 2 © h1 · ¸ ¸ ¹ V12 2 'E system Ê0 (steady) 0 Rate of change in internal, kinetic, potential, etc. energies E out § V2 · m ¨ h2 2 ¸) ¨ 2 ¸¹ © h2 + 700 kPa 100qC 30 m/s Air 200 kPa V 22 2 Solving for the exit velocity, V2 >V >V @ 0.5 2 1 2(h1 h2 ) 2 1 2c p (T1 T2 ) @ 0.5 ª § 1000 m 2 /s 2 «(30 m/s) 2 2(1.005 kJ/kg K)(373 279)K¨¨ «¬ © 1 kJ/kg 436 m/s ·º ¸» ¸» ¹¼ 0.5 PROPRIETARY MATERIAL. © 2008 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.