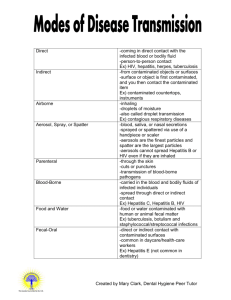
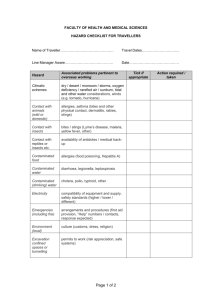
Exposure Control Plan for Blood-borne Pathogens
advertisement

REDWOODS COMMUNITY COLLEGE DISTRICT Exposure Control Plan for Blood-borne Pathogens Table of Contents BACKGROUND ..................................................................................................................... 1 INTRODUCTION ............................................................................................................................... 2 OVERVIEW OF BLOOD-BORNE PATHOGENS ....................................................................... 3 EMPLOYEES AND STUDENTS AT RISK ........................................................................5 METHODS OF COMPLIANCE AND SCHEDULE OF IMPLEMENTATION ........................ 6 PERSONAL PROTECTIVE EQUIPMENT .................................................................................... 8 CUSTODIAL ........................................................................................................................... 9 LAUNDRY ........................................................................................................................................ 10 HEPATITIS B IMMUNIZATION PLAN AND WAIVER PROCESS...................................... 11 POST-EXPOSURE EVALUATION AND FOLLOW-UP ...........................................................12 COMMUNICATION OF HAZARDS TO EMPLOYEES AND STUDENTS ..........................15 INFORMATION AND TRAINING ...............................................................................................16 RECORD-KEEPING .........................................................................................................................18 GLOSSARY ....................................................................................................................................... 19 INTERFACE WITH OTHER STANDARDS ............................................................................... 23 MANAGEMENT OF MEDICAL WASTE ................................................................................... 24 APPENDICES .................................................................................................................................. 25 i APPENDIX A, Sample Forms ........................................................................................... 26 APPENDIX B, Blood-borne Pathogens Standard, Title 8, California Code of Regulations Section 5193 ........................................................................................................ 39 APPENDIX C, Most Frequently Asked Questions about the Blood-borne Pathogen Standard ............................................................................................... 40 APPENDIX D, California Medical Waste Act ............................................................... 41 APPENDIX E, Resources for and Information on HIV IAIDS and Hepatitis B ............ 42 APPENDIX F, Exposure Determination Worksheets ..................................................... 43 APPENDIX G, Exposure Control Plan Checklist .......................................................... 45 APPENDIX H, Signs and Labels ....................................................................................... 46 APPENDIX I, Sharps Containers and Other Contaminated Material Containers ... 52 APPENDIX J, Preservation of Employee's Records ........................................................ 56 APPENDIX K, Labeling Requirements ............................................................................. 58 BACKGROUND In September 1986, OSHA was petitioned by various unions representing healthcare employees to develop an emergency temporary standard to protect employees from occupational exposure to blood-borne diseases. The agency decided to pursue the development of 8CCR Section 5 1 9 3 . 1 INTRODUCTION The Redwoods Community College District (hereafter referred to as the District) is implementing an Exposure Control Plan to ensure the well-being of and to protect the safety and health of our employees and students. This plan has been developed to comply with state and federal regulations pertaining to blood-borne pathogens and communicable diseases. Employees and students are encouraged to read and are required to follow the guidelines and procedures set forth in this plan. Questions regarding the contents of this plan should be brought to your immediate supervisor or classroom instructor. A copy of this plan can be found on the College’s web site. 2 OVERVIEW OF BLOOD-BORNE PATHOGENS HEPATITS Hepatitis has been categorized into several distinct forms. Hepatitis A accounted f o r approximately 28,500 cases in 1988. Hepatitis B was reported 23,200 times; and 2,620 cases of non-A/non-B hepatitis were reported, as well as 2,470 cases of unspecified diagnoses. The United States Public Health Services (USPHS) believes that the actual number of infections is many times the reported n u m b e r . Hepatitis A Hepatitis A is a viral infection caused by a picornavirus, is commonly transmitted by the fecal-oral route, and is generally controlled through good hand-washing techniques. NonA/Non-B Hepatitis (Hepatitis C/Hepatitis E) Non-A/non-B hepatitis has been shown to be transmitted by the fecal-oral route and parenterally. Parenterally transmitted, non-A/non-B hepatitis can make up as much as 40 percent of the acute viral hepatitis in the United States. Transfusion patients and parental drug users are considered the groups most at risk. Healthcare staff who frequently works with blood may be at risk. However, little is known about person-to-person transmission of this disease. Hepatitis B Virus (HBV) The hepatitis B infection is caused by a specific virus known as a DNA virus. The incubation period can be as long as 160 days, with an average of 120. The symptoms and signs include anorexia, malaise, nausea, vomiting, abdominal pain, and jaundice. Chronic carriers of the disease are common. The chronic stage of the disease is more common in the younger individuals. The carrier is capable of passing the disease to others. The body fluids containing the highest concentrations of the virus are the blood and blood fluids. The potential risk for workers handling these fluids is obvious. Although not transmitted through the fecal-oral route, HBV is commonly transmitted through the use of contaminated needles or sexual contact. Transmission through blood transfusion is rare only because of donor and blood supply screening. Transmission through close personal contact can also occur. Although about 300,000 people in the United States are infected with the virus annually, as many as 1,000,000 may be carriers of the disease. Workers exposed to infected blood are the most at risk. The USPHS lists those at highest risk as medical and dental employees and staff in institutions and classrooms for the mentally retarded. Vaccines are available for prevention and post-exposure situations. 3 HIV/AIDS HIV is transmitted through sexual contact and exposure to infected blood. Although the virus has been isolated from many body fluids, it is known to be transmitted through contact with blood, semen, and vaginal secretions. The reservoir of infection in the United States has surpassed 1,500,000 people and is increasing as more become infected annually. Therefore, the potential risk for the healthcare worker is probably increasing. 4 EMPLOYEES AND STUDENTS AT RISK The District has determined that the following job classifications and responsibilities include employees with potential occupational exposure: • • • • • • • • • • • • • • • • • • Physicians Registered nurses Nurse practitioners Employees in clinical and diagnostic labs Freestanding clinics (e.g. family planning clinics, urgent care clinics) Dentists Dental hygienists and assistants Dental laboratory personnel Custodians and other employees handling regulated waste Medical or dental equipment service personnel (maintenance mechanics) Emergency response personnel (e.g. EMT, paramedics) Campus security personnel Physical education instructors Coaches Trainers Health occupations personnel Early Childhood Education/Child Development Center personnel Sewer plant operators and plumbers Other Employee Situations Covered by this Plan • • Part-time, temporary, and student employees. Employees trained in first aid who are expected or authorized to render first aid in an emergency situation as part of their job duties. Students Covered by this Plan • • • Health Occupations majors Students enrolled in life science lab courses Early Childhood Education majors 5 METHODS OF COMPLIANCE AND SCHEDULE OF IMPLEMENTATION Universal precautions will be observed to prevent contact with blood or other potentially infectious materials (OPIM). Under circumstances in which differentiation between body fluid types is difficult or impossible, all body fluids shall be considered potentially infectious materials. Engineering and Workplace Controls The following engineering and workplace controls shall be used to eliminate or minimize employee exposure: • ENGINEERING CONTROLS: • HAND-WASHING FACILITIES: These shall be readily available. Hand-washing will be done These shall be maintained on a regular schedule. A regular system will include documentation of maintenance inspection, data, employee making the inspection, findings, repair verification if needed, and signature of employee conducting the inspection. as soon after hand contamination as possible. If water is not available, an antiseptic hand cleaner must be used with clean cloth, paper towels, or antiseptic towelettes. • SHEARING AND BREAKING: Shearing and breaking of contaminated needles is prohibited. • BENDING, RECAPPING, OR REMOVAL: Bending, recapping, or removal of contaminated needles by hand is prohibited. • REUSABLE • The area where employees eat and drink must be separated from contaminated work areas and clothing by a partition. • CONTAMINATED CLOTHING AND EQUIPMENT: SHARPs: These pose the same exposure hazard as disposable sharps. Therefore, their containers will meet the same requirements. CONTAMINATED ENVIRONMENTAL SURFACES AND WORK AREAS: Must be removed before entering a food consumption area. • Splattering or the generation of droplets or aerosols of contaminated material must be avoided. Where such a potential exists, face protection will be required. • CONTAMINATED REUSABLE EQUIPMENT: SPLATI'ERING OR THE GENERATION OF DROPLETS OR AEROSOLS: Must be decontaminated to the extent possible by employees with appropriate personal protective equipment. 6 • PERSONAL PROTECTIVE CLOTHING: Must be worn to prevent body contamination and shall be provided by the District. • PERSONAL PROTECTIVE EQUIPMENT (SPLASH SHIELDS, CLOTHES, GWVES, ETC.): not be taken home by the employee and should remain at work. • SPLASHES ON PROTECTIVE CLOTHING: 7 Should be inspected for soak-through. Must PERSONAL PROTECTIVE EQUIPMENT 1. The District shall make personal protective gloves available to employees and students in high-risk areas. 2. Because not all gloves are completely impermeable, hand-washing after glove removal is required. 3. Gloves shall be inspected for wear and discarded should the integrity of the glove barrier be compromised. 4. Disposable gloves shall never be reused. 5. The Human Resources Office and the Facilities Director, Maintenance & Operations will analyze employee and student tasks and the type of exposure expected in order to select personal protective clothing and equipment that will provide adequate protection. These items may include gowns, aprons/laboratory coats, clinic jackets, surgical caps, and shoe covers. This will be accomplished in view of the fact that there is no standardized method of testing and classification of the resistance of clothing to biological hazards. 6. Employees will be required to wear designated, required, protective equipment while on duty. 7. Students without designated, required, protective equipment will not be allowed to participate in the class. 8 CUSTODIAL 1. The District will develop a Schedule of Disinfection for any work surface that may become contaminated by the HIV or HBV virus. The type of sterilant utilized will be found on the EPA list of Registered Sterilants and shall be approved for the highest antimicrobial activity in order to kill HIV and the more resilient HBV virus. 2. Protective covering will be replaced as soon as it is feasible. 3. Broken glassware that may be contaminated will not be picked up with bare hands, nor shall any employee reach into a container of broken glassware. 4. Regulated waste includes the following categories: • • • • • liquid blood or other potentially infected material (OPIM), items contaminated with blood or OPIM which would release the blood if squeezed or shaken, items caked with dried blood or OPIM which could be released if handled, contaminated sharps, and pathological and microbiological wastes. 5. Sharps containers will be designed according to regulations, will not be allowed to overfill, and will be located so that employees will not have to walk long distances with used syringes. Disposable sharps containers are recommended. 6. Sharps containers will be inspected regularly according to the department administrator and will be replaced as required. 7. Other waste containers shall be of a capacity to hold the volume of waste generated between scheduled pickups. 8. All containers will be inspected for leakage potential. Secondary containers will be available if leakage is possible. 9. All containers holding contaminated material will comply with CCR, Title 8, Chapter 4. (See Appendix H .) 10. Removal of regulated waste will be performed in compliance with OSHA Instruction CPL 2-2.44B. Performance of removal activities shall be the responsibility of the Director, Maintenance & Operations of Facilities and Grounds. 9 LAUNDRY 1. Contaminated laundry will not be sorted or rinsed at the location of use. 2. Contaminated laundry will be bagged by employees utilizing proper personal protective equipment and bagged with consideration for outside contamination and proper labeling. 3. When contaminated laundry is shipped off site, it should be noted that the receiving facility may not practice Universal Precautions. Proper labeling should reflect this according to relevant regulations. Title 8: CCR Section 5193(d)(4)(D) a. Contaminated laundry shall be handled as little as possible with a minimum of agitation. 1) Contaminated laundry shall be bagged or containerized at the location where it was used and shall not be sorted or rinsed at the location of use. 2) Contaminated laundry shall be placed and transported in bags or containers labeled and color-coded in accordance with Subsection (g) (a) (A) of this standard. When a facility utilizes Universal Precautions in the handling of all soiled laundry, alternative labeling and color-coding is sufficient if it permits all employees and students to recognized the containers as requiring compliance with Universal Precautions. 3) Whenever contaminated laundry is wet and presents a reasonable likelihood of soak-through or of leakage from the bag or container, the laundry shall be placed and transported in bags or containers that prevent soak-through and/or leakage of fluids to the exterior. b. The employer shall ensure that employees and students who have contact with contaminated laundry wear protective gloves and other appropriate personal protective equipment. c. When a facility ships contaminated laundry off site to a second facility that does not utilize Universal Precautions in the handling of all laundry, the facility generating the contaminated laundry must place such laundry in bags or containers that are labeled and color-coded in accordance with Subsection (g)(1)(A). 10 HEPATITIS B IMMUNIZATION PLAN AND WAIVER PROCESS 1. The Hepatitis B vaccination series shall be made available by the employer to all employees and students with occupational exposure. In addition, a post-exposure evaluation and follow-up will be made available to all employees who are exposed to the HBV. 2. The employee will contact the Human Resources Office and the Student Health Center for any of these services. 3. The District shall follow the regulations as stated in CCR, Title 8, Chapter 4, Section 5193 (f) concerning the management of the vaccination and follow-up programs. 4. The basic requirements are that the vaccination and post-exposure evaluation and follow- up, including prophylaxis, are as follows: • • • • • available at no cost to the employee, available at a reasonable time and place, under the supervision of a licensed physician or other licensed healthcare worker, provided according to the recommendations of the USPHS, and all lab tests will be conducted by an accredited laboratory. 5. Employees identified in the Exposure Control Plan shall be required to receive the hepatitis B vaccine. The vaccine will be provided through the college health services at no cost to the employee. 6. Students identified in the Exposure Control Plan shall be required to obtain the hepatitis B vaccine. The vaccine will be available through the college health services at a reduced rate, payable by the student. 7. Any at-risk employee or student refusing the vaccine must sign the approved waiver form. (See Appendix A, Vaccination Declination Form.) 11 POST-EXPOSURE E V A L U A T I O N AND FOLLOW-UP The District realizes the importance of the follow-up and evaluation of HIV and HBV exposure incidents. The District will, therefore, follow precisely the regulation as stated below: 1. Following a report of an exposure incident, the employer shall make immediately available to the exposed employee or student a confidential medical evaluation and follow-up, including at least the following elements: a) Documentation of the route(s) of exposure and the circumstances under which the exposure incident occurred. b) Identification and documentation of the source individual, unless the employer can establish that identification is unfeasible or prohibited by state or local law. c) • The source individual's blood shall be tested as soon as feasible and after consent is obtained in order to determine HBV and HIV infectivity. If consent is not obtained, the employer shall establish that legally required consent cannot be obtained. When the source individual's consent is not required by law, the source individual's blood, if available, shall be tested and the results documented. • When the source individual is already known to be infected with HBV or HIV, testing for the source individual's known HBV or HIV status need not be repeated. • Results of the source individual's testing shall be made available to the exposed employee, and the employee shall be informed of applicable laws and regulations concerning disclosure of the identity and infectious status of the source individual. Collection and testing of blood for HBV and HIV serological status. • The exposed employee's or student's blood shall be collected as soon as feasible and tested after consent is obtained. • If the employee or student consents to baseline blood collection but does not give consent at that time for HIV serologic testing, the sample shall be preserved for at least 90 days. If, within 90 days of the exposure incident, the employee or student elects to have the baseline sample tested, such testing shall be performed as soon as feasible. 12 • 2. Additional collection and testing shall be made available as recommended by the U.S. Public Health Service. d) Post-exposure prophylaxis, when medically indicated, as recommended by the U.S. Public Health Service. e) Counseling f) Evaluation of reported illnesses. INFORMATION PROVIDED TO THE HEALTHCARE PROFESSIONAL a) The employer shall ensure that the healthcare professional responsible for the employee's or student's hepatitis B vaccination is provided a copy of the regulation(s). b) The employer shall ensure that the healthcare professional evaluating an employee or student after an exposure incident is provided the following information: c) • A copy of this regulation • A description of the exposed employee's duties as they relate to the exposure incident, or description of the exposed student's duties related to the exposure incident. • Documentation of the route(s) of exposure and circumstances under which exposure occurred, as required by Subsection (f)(3)(A) • Results of the source individual's blood testing, if available • All medical records relevant to the appropriate treatment of the employee or student, including vaccination status, which are the employer's responsibility to maintain as required by Subsection (h)(l)(B)2. Healthcare Professional's Written Opinion The employer shall obtain and provide the employee or student with a copy of the evaluating healthcare professional's written opinion within 15 days of the completion of the evaluation. 13 The healthcare professional's written opinion for hepatitis B vaccination shall be limited to whether hepatitis B vaccination is indicated for an employee or student and if the employee or student has received such vaccination. The healthcare professional's written opinion for post-exposure evaluation and follow-up shall be limited to the following information: • The healthcare professional’s written opinion for hep B vaccination shall be limited to whether hep B vaccination is indicated for an employee or student has received such vaccination. • The healthcare professional’s written opinion for post exposure evaluation and follow-up shall be limited to the following information” • The employee or student has been informed of the results of the evaluation • The employee or student has been informed of any medical conditions resulting from exposure to blood or other potentially infectious materials that require further evaluation or treatment. All other findings or diagnoses shall remain confidential and shall not be included in the written report. 14 COMMUNICATION OF HAZARDS TO EMPLOYEES AND STUDENTS LABELS AND SIGNS 1. Warning labels will be placed on refrigerators and freezers containing blood or other potentially infectious materials. 2. Labels shall comply with Title 8, Section 6004, and Health and Safety Code Sections 25080-25082. (See Appendix H, Signs and Labels, and Appendix I, Sharps Containers and O her Contaminated Material Containers.) 3. Labels concerning biohazardous waste are covered in Health and Safety Code, Sections 25080-25082. Color coding is described in Title 8, Section 6003. (See Appendix H, Signs and Labels.) 4. The District shall post signs at the entrance to work areas as described in the regulation. (See Appendix H, Signs and Labels.) 15 INFORMATION AND TRAINING 1. 2. The Human Resources Office and the Student Health Office will provide training to all employees with occupational exposure as follows: • Training will occur at the time a new employee comes on board and at least annually. • Retraining will occur as operations change affecting exposure. • The programs will be provided at no cost and will be delivered during work hours. • The content of the training will be appropriate for the educational level of the employee. The content of the training will include the following topics: • An explanation of the Blood-borne Pathogens Standard; • Blood-borne disease epidemiology and symptoms; • Modes of transmission; • Recognition of tasks and activities that expose employees to the viruses; • The use and limitations of engineering controls, personal protective equipment, and work practices; • Types, use, location, removal, handling, and decontamination of personal protective equipment; • How to select personal protective equipment; • Information on the hepatitis B vaccine; • Handling emergencies involving blood or other potentially infectious materials; • Exposure incident procedures and reporting; • Information on post-exposure follow-up and evaluation; • Signs, labels, and other warnings; and 16 • Questions and other interaction. 3. The training for students in classes where exposure is possible shall be provided by the instructor of record in the class in which the student is enrolled. 4. The content of the training will include the following topics: • An explanation of the Blood-borne Pathogens Standard; • Blood-borne disease epidemiology and symptoms; • Modes of transmission; • Recognition of tasks and activities that expose students to the viruses; • The use and limitations of engineering controls, personal protective equipment, and work practices; • Types, selection, and use of personnel protective equipment; • Universal Precautions; • Exposure incident procedures and reporting; and • Questions and other interaction. 17 RECORD-KEEPING The Human Resources Office will maintain accurate records on occupational exposure of each employee, and the Student Health Office will maintain accurate records on occupational exposure of students pursuant to CCR 8 Section 3204 (d). (See Appendix J, Preservation of Records.) These records will be confidential and will be released only by the employee's or student's written permission or as required by law or regulation. The records will be maintained for thirty years beyond the end of employment of the employee and thirty years beyond the end of enrollment of the student. CONTENT OF RECORDS • Name and social security number of employees or students • Copies of HBV vaccination status and other relevant records • Copies of results of medical exams, testing, and follow-up • Employer's copy of healthcare professional's written opinion as required in the regulation • Copy of the information provided to the healthcare professional as required in the regulation TRAINING RECORDS • Dates of training sessions • Content summary of training • Names and qualifications of trainers • Names of job titles of all employees attending the training session • Names and course title of students enrolled in life science lab and health occupations courses 18 GLOSSARY BIOLOGICALCABINET A device enclosed, except for necessary exhaust purposes, on three sides and top and bottom, designed to draw air inward by means of mechanical ventilation, operated with insertion of only the hands and arms of the user, and in which virulent pathogens are used. Biological cabinets are classified as follows: CLASS 1 A ventilated cabinet for personnel protection with an un-recirculated inward airflow away from the operator and HEPA filtered exhaust air for environmental protection. CLASS 2 A ventilated cabinet for personnel, product, and environmental protection having an open front with inward airflow for personnel protection, HEPA filtered laminar airflow for product protection, and HEPA filtered exhaust air for environmental protection. CLASS 3 A totally enclosed, ventilated cabinet of gas-tight construction. Operations in the cabinet are conducted through attached, protective gloves. BLOO Meaning human blood, human blood components, and products made from human blood. BLOOD-BORNE PATHOGENS Pathogenic microorganisms that are present in human blood and that can cause disease in humans. These pathogens include, but are not limited to, HBV and HIV. CHIEF The Chief of the Division of Occupational Safety and Health of the California Department of Industrial Relations, or designated representative. CLINICAL LABORATORY A workplace where diagnostic or other screening procedures are performed on blood or other potentially infectious materials. CONTAMINATE The presence, o r the reasonably anticipated presence, of blood or other potentially infectious materials on a surface or in or on an item. 19 CONTAMINATED LAUNDRY Soiled with blood or other potentially infectious materials, or may contain sharps. CONTAMINATED SHARPS Any contaminated object that can penetrate the skin, including, but not limited to, needles, scalpels, broken glass, broken capillary tubes, and exposed ends of dental wires. DECONTAMINATION The use of physical or chemical means to remove, inactivate, or destroy blood-borne pathogens on a surface or site to the point where they are no longer capable of transmitting infectious particles and the surface or item is rendered safe for handling, use, or disposal. Decontamination includes procedures regulated by Health and Safety Code Section 25090. EMERGENCY RESPONSE The response by employees, who are designated by their employer as emergency response personnel, to fire, accident, earthquake, explosion, or other incidents. ENGINEERING CONTROLS Controls (e.g. sharps disposal containers, self-sheathing needles) that isolate or remove the bloodborne pathogens hazard from the workplace. EXPOSURE INCIDENT A specific eye, mouth, other mucous membrane, non-intact skin, or parenteral contact with blood or other potentially infectious materials that results from the performance of an employee's duties. HAND-WASHING FACILITIES A facility providing an adequate supply of running, potable water, soap, and single-use towels or hot-air drying machines. HBV Hepatitis B virus. HV Human immunodeficiency virus. LICENSED HEALTHCARE PROFESSIONAL A person whose legally permitted scope of practice allows him or her to independently perform the activities required by Subsection (t), hepatitis B vaccination, and post-exposure evaluation and follow-up. 20 NIOSH The National Institute for Occupational Safety and Health, United States Department of Health and Human Services. OCCUPATIONAL EXPOSURE Reasonably anticipated skin, eye, mucous membrane, or parenteral contact with blood or other potentially infectious materials that may result from the performance of any employee's duties. ONE-HAND TECHNIQUE Procedure wherein the needle of a reusable syringe is capped in a sterile manner during use. The technique employed shall require the use of only the hand holding the syringe, so the free hand is not exposed to the uncapped needle. OTHER POTENTIALLY INFECTIOUS MATERIALS • The following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardia! fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any other body fluid that is visibly contaminated with blood such as saliva or vomitus, and all body fluids in situations such as emergency response, where it is difficult or impossible to differentiate between body fluids. • Any unfixed tissue or organ (other than intact skin) from a human (living or dead). • HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV. PARENTERAL Piercing mucous membranes or the skin barrier through such events as needle-sticks, human bites, cuts, and abrasions. PERSONAL PROTECTIVE EQUIPMENT Specialized clothing or equipment worn by an employee for protection against a hazard. General work clothes (e.g. uniforms, pants, shirts, or blouses) not intended to function as protection against a hazard are not considered to be personal protective equipment. PRODUCTION FACILITY A facility engaged in industrial-scale, large-volume, or high-concentration production of HIV or HBV. REGULATED WASTE Liquid or semi-liquid blood or other potentially infectious materials; contaminated items that would release blood or semiliquid state if compressed; items that are caked with dried blood or other potentially infectious materials and are capable of releasing these materials during handling; 21 contaminated sharps; and pathological and microbiological wastes containing blood or other potentially infectious materials. Regulated waste includes "medical waste" regulated by Health and Safety Code, Chapter 6.1. RESEARCH LABORATORY A laboratory producing or using research-laboratory-scale amounts of HIV or HBV. Research laboratories may produce high concentrations of HIV or HBV but not in the volume found in production facilities. SOURCE INDIVIDUAL Any individual, living or dead, whose blood or other potentially infectious materials may be a source of occupational exposure to the employee. Examples include, but are not limited to, hospital and clinic patients, clients in institutions for the developmentally disabled, trauma victims, clients of drug and alcohol treatment facilities, residents of hospices and nursing homes, human remains, and individuals who donate or sell blood or blood components. STERILIZE The use of a physical or chemical procedure to destroy all microbial life, including highly resistant bacterial endospores. Sterilization includes procedures regulated by Health and Safety Code Section 25090. UNIVERSAL PRECAUTIONS An approach to infection control. According to the concept of Universal Precautions, all human blood and certain human body fluids are treated as if known to be infectious for HIV, HBV, and other blood-borne pathogens. WORK PRACTICE CONTROLS Controls that reduce the likelihood of exposure by altering the manner in which a task is performed (e.g., prohibiting recapping of needles by two-handed techniques). 22 INTERFACE WITH OTHER STANDARDS The District recognizes that this plan must work with other regulations. The following statements indicate how the District will comply with other similar requirements found in other regulations. • The Hazard Communication Standard, Title 8 Section 5194, applies only to chemicals in the workplace and does not apply to biological hazards such as blood-borne disease. • Records concerning employee exposure to blood-borne pathogens and records about HIV and HBV status are employee medical records according to 29 CPR 1910.20. • The Respiratory Protection Standard, 29 CPR 1910.134, does not apply since no respirators are approved for biohazards. Nevertheless, r e s p i r a t o r s may not be placed where they can be contaminated by body fluids. • The federal hazardous waste operations and emergency response standard includes workers expected to respond to emergencies caused by the uncontrolled release of a hazardous substance. The definition of hazardous substance includes biological agent or infectious materials. Consequently, workers responding to emergencies caused by a release of infectious material will receive appropriate HAZWOPER Training. 23 MANAGEMENT OF MEDICAL WASTE As a result of federal legislation, the California Infectious Waste regulations have been revised. The California Medical Wastes Management Act provides for many changes to the old regulation. The following briefly describes the provisions which may impact the Infection Control Plan for blood-borne pathogens. According to the 1990 California Medical Wastes Management Act, medical wastes include materials classified as biohazards or sharps waste. Medical wastes may be generated as a result of medical treatment, diagnosis, or immunization. Biohazardous waste included in the Blood-borne Pathogens Infection Control Plan is medical waste and, therefore, will be regulated by the Waste Management Act. Sharps waste also described in the Blood-borne Pathogens Infection Control Plan is regulated by the Medical Waste Management Act. This includes items having rigid comers, edges, or protuberances capable of cutting or piercing. Examples include knives, needles, syringes, and broken glassware. Large Quantity Generators (LQGs) of medical waste include those facilities generating more than 200 pounds per month. LQGs must prepare a Medical Waste Management Plan and develop methods for storage, containment, and treatment. Record-keeping is also required. Most school facilities would be classified as Small Quantity Generators (SQGs). SQGs must register with the State Department of Health Services if they use any on-site treatment methods. If the facility treats waste on site, they must develop a Medical Waste Management Plan and include medical waste treatment and tracking records. Those SQGs not treating waste on site must prepare information on the storage, treatment, and disposal of medical waste and prepare medical waste transport records as described in the Health and Safety Code Sections 25046 through 25047. Medical Waste must be transported by a licensed hazardous waste hauler unless the generator has a limited quantity exemption. 24 APPENDICES Appendix A Sample Forms Appendix B Blood-borne Pathogens Standard, Title i, California Code of Regulations Section 5193 Appendix C Most Frequently Asked Questions about the Blood-borne Pathogen Standard Appendix D California Medical Waste Act Appendix E Resources for and Information on HIVIAIDS and Hepatitis B Appendix F Exposure Determination Worksheets Appendix G Exposure Control Plan Checklist Appendix H Signs and Labels Appendix I Sharps Containers and Other Contaminated Material Containers Appendix J Preservation of Employee's Records Appendix K Labeling Requirements Reviewed: June 2015 25 APPENDIX A Sample forms included in Appendix A: Employees Eligible for Hepatitis B Vaccination Informed Consent Signature Form Vaccination Declination Form Hepatitis B Vaccination Status Form Exposure Determination Work Sheet Record of Blood-borne Pathogens Exposure and Treatment Blood-borne Pathogens Training Documentation Source Individual Consent Form Authorization for Disclosure by Health Care Provider of the Results of the Source Individual Blood Test Authorization for Disclosure by School District of the Results of the Source Individual Blood Test Occupational Exposure Incident Report Form Post-exposure Evaluation and Follow-up Procedures 26