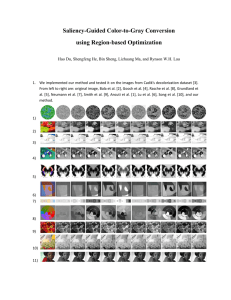
Saurashtra University ,
advertisement

Saurashtra University Re – Accredited Grade ‘B’ by NAAC (CGPA 2.93) Shrivastava, Rohit S., 2005, “Enzymology of Degradation of Xenobiotic Compounds by Lignin-Degrading Fungi”, thesis PhD, Saurashtra University http://etheses.saurashtrauniversity.edu/id/eprint/600 Copyright and moral rights for this thesis are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the Author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the Author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Saurashtra University Theses Service http://etheses.saurashtrauniversity.edu repository@sauuni.ernet.in © The Author A Thesis Submitted for the Degree of Doctor of Philosophy In Microbiology ENZYMOLOGY OF DEGRADATION OF XENOBIOTIC COMPOUNDS BY LIGNIN- DEGRADING F UNGI Rohit S hrivastava Registration No: 2594 Date: June 12, 2001 Department of Bioscience s, Saurashtra University, Rajkot 360 005 , Gujarat, India. January, 2005 Think no more, lad; laugh, be jolly: Why should men make haste to die? Empty heads and tongues a -talking Make the rough road easy walking, And the feather pate of folly Bears the falling sky. Oh, 'tis jesting, dancing, drinking Spins the heavy world around. If young hearts were not so clever, Oh, they would be young for ever: Think no more; 'tis only thinking Lays lads underground. A. E. Housman A Shropshire Lad, (1896) Dedicated to . . . . . . . . . . . My Beloved Parents SAURASHTRA UNIVERSITY DEPARTMENT OF BIOSCIENCES University Campus, RAJKOT – 360 005, Gujarat, India Phone: (O) +91-281–2586419, (R) 2444295 Fax : 2586419 E-mail : brmvyas@hotmail.com Dr. B.R.M. Vyas Assistant Professor Date: January , 2005 Ref: SU/BIO/ C ERTIFICATE I take pleasure in forwarding the thesis entitled “Enzymology of Degradation of Xenobiotic Compounds by Lignin-degrading Fungi” of Mr. Rohit Shrivastava for the acceptance of the degree of Doctor of Philosophy in Microbiology. Thesis presented here embodies a record of the results of original investigation carried out by him. (B. R. M. Vyas) Forwarded Through: Dr. S. P. Singh Professor & Head Dept. of Biosciences Saurashtra University A CKNOWLEDGEMENTS “Acknowledgement” – This is where one admits, the imminence, involvement and dedication of others to your work. It is indeed a moment of great pleasure to mention the names of those wonderful persons who walked-along till my thesis. Firstly, my Supervisor, Dr. B.R.M. Vyas, a niche of its own, whose enthusiastic cry of “Any Progress” will haunt me for the rest of my days - his unquenchable curiosity and love for the subject are probably the most valuable lessons I have learnt from this PhD. I feel sacred, for He provided the creative and stimulating atmosphere that kept me going over the last four years and lent a hand to shape-up my research to the existing dimension. I am grateful to Prof. S.P. Singh ( Head, Department of Biosciences) and Prof. P.P. Sood (Former Head, Department of Biosciences) for providing me the laboratory, needed facilities and for extending overwhelming enthusiasm during my course of research . Their ideology and approach helped to prepare me for life, both Inside & Outside my profession. I pay my sincere gratitude to Dr. S.J. Pathak and Dr. S.C. Bhatt for their continual assistance, encouragement and whole-hearted support for whenever I needed during the investigation. My thanks are due to all the Non-Teaching Staff, Parekhbhai, Rajubhai, Zalabhai, Thakrarbhai & Nitesh for their kind co-operation. I gratefully acknowledge Department of Biotechnology (DBT), Ministry of Science & Technology, India, for the financial support for my Senior Research Fellowship. I salute my Guru-of-the-Field, Dr. P.S. Nagar (whom we use d-to call “Baba” with love), sagacity personified. The first harvest with him taught me the real importance of other living things, the work outside the fourwalls and to respect the work for them. I gratefully acknowledge my Laboratory-Colleagues Pinalee, Jagruti, Dharmendra , Padma & Charmy for their help that rendered the completion of investigation. No phrases can fully express my gratitude to My-Dear-Labmates Kalpesh ( Batti), Vimesh (Vimli), & Dr. Rajendra (Chacha) for their extraordinary support, especially during my experiments when we used to move like Night-Hawkers to stay awaken through -out the completion of experiment. I owe my sincere debt to my Small-F amily, Viral (Viraliya), Sudipta (Misra), Mital (Mitu), & Rupal (Rupali) for realizing me, what it means to be, home away from home that made my life, A –Phenomenon, at Rajkot. The “Luncheon” was always exotic for the meals and the moments shared. I run short of words to express my hearty-thanks for their selfless dedication, untiring efforts and magical-warm-caress that made the rough road easy walking en-route to the final milestone. Thanks to my Friends, Nikhil (Pange), Rahul (Baccha), Ajay (Chowdhary), Nirav (Nirva), & Ranjeet (Paaji), for their invariable propup in organizing my administrative work at Ahmedabad. Finally, my deepest gratitude with homage to my Loving-Family, Dr. S.M. Shrivastava (Papa), Mrs. Punam Shrivastava (Ma’) & Ms. Nupur Shrivastava (Pinky), who have been great over the years and never raised an eyebrow when I claimed my thesis, would be finished in the “next two months” for nearly a year.. .. .. .. .. .. .. Rohit Shrivastava C ONTENTS Page No. Chapter 1. Introduction 1-25 (A) White-Rot Basidiomycetes: Lignin Degradation 1-14 (B) White-Rot Basidiomycetes: Degradation of Dyes 15-25 Chapter 2. Screening of White-Rot Basidiomycetes: Decolorization 26-32 of Textile dyes 2.1 Introduction 2.2 Materials and methods 26 27 2.3 Results and discussion 28-32 Chapter 3 . Ligninolytic Enzymes of Irpex lacteus: Decolorization 33-41 of Textile and Triphenyl methane Dyes 3.1 Introduction 33 3.2 Materials and methods 3.3 Results and discussion 34-35 35-41 Chapter 4. Enzymatic decolorization of Sulfonphthalein Dyes 42-52 4.1 Introduction 4.2 Materials and methods 42 43-44 4.3 Results and discussion 44-52 Chapter 5. Horseradish Peroxidase: Decolorization of Textile And 53-59 Triphenyl methane Dyes 5.1 Introduction 53 5.2 Materials and methods 5.3 Results and discussion 54 54-59 Chapter 6. Ligninolytic Enzymes of Irpex lacteus : Decolorization 60-67 of Effluents 6.1 Introduction 60 6.2 Materials and methods 6.3 Results and discussion 61-62 62-67 Chapter 7. Decolorization of D yes and Effluents by Fenton 68-75 and Fenton-like R eagent 7.1 Introduction 7.2 Materials and methods 68 69 7.3 Results and discussion 70-75 BIBLIOGRAPHY IMPLICATIONS AND SPECULATIONS OF INVESTIGATIONS APPENDIX 76-95 96-99 100-106 I. Scholarships awarded 100 II. List of Publications & Presentations 101-104 III. Conferences / Seminars / Workshops attended 105-106 CHAPTER 1a WHITE-R OT BASIDIOMYCETES: D EGRADATION OF LIGNIN L ignum in Latin means wood. It is found in higher plants, including ferns, but not in liverworts, mosses and plants of lower taxa. Lignin differs from plant to plant, in different parts of plant and in different age groups of plant. Lignin is therefore not a single compound but a group of compounds. It is estimated that our planet contains 3x10 11 metric tones of lignin with an annual biosynthesis rate of approximately 30% of the dry weight of softwoods and 20% of the weight of hardwoods. Lignification is associated with the development of vascular system in plants. Lignin biosynthesis: Lignin is built from phenyl propane units linked together by several different inter-unit linkages having bio logically stable structure. Biosynthesis of lignin initiates with the fixation of CO2 by plants leading to the generation of phenylalanine from glucose via shikimic acid pathway (1). Lignification proceeds with the enzymatic conversion of phenylalanine to cinnamic acid which is hydroxylated to coumaric acid and caffeic acid. Methylation of caffeic acid sequentially yields ferulic acid and sinnapic acid. The synthesis of lignin precursors completes with the reduction of coumaric acid, ferulic acid, and sinnapic acid to their respective alcohols (Figure 1.1). CH2OH CH2OH CH2OH C C C C C C OH Coumaryl alcohol OH Coniferyl alcohol OCH3 OCH3 OCH3 OH Sinnapyl alcohol Figure 1.1 Precursors of lignin biosynthesis 1 These monolignols are of low solubility and are stabilized in cell wall as their glucosides. The polymerization of monolignols initiates with the dehydrogenation catalyzed by peroxidase-H2O2 or laccase-O2 system generating spontaneous resonance and stabilized random radicals polymerization (1). The reactions of non-enzymatic, enzymatically- generated radicals completes the three dimensional network of lignin. Lignin structure and its role: The structural and physico-chemical properties (Table 1.1) make lignin a highly recalcitrant polymer that resists degradation in nature. Lignin is a natural bio-plastic that (i) provides mechanical rigidity and strength to stem, (ii) water proofs cell wall and thereby reduces water-loss occurring through evaporation, and also enables transport through xylem and phloem, (iii) protects plants against UV-rays due to its ability to absorb UVradiations, (iv) protects plants against microbial attack, and (v) plays an important role in healing as injury to plant induces lignification. Table 1.1 Physical and chemical properties of lignin Physical Properties Water insoluble High molecular weight Highly branched 3D structure Compact Random Optically inactive Stereo-irregular Chemical Properties Highly reduced form of carbon Presence of chiral C -atoms Linkages Ø Variety of linkages Ø Non-hydrolysable ether bonds (β-o-4, α-o-4, etc.) Ø Multiple linkages (phenylcoumaran, pinoresinol, etc.) Ø Randomly distributed linkages Various microbial groups possess the ability to degrade lignin but white rot basidiomycetes comprise the only group of organisms known to degrade lignin completely (2). The degradation of lignin by white rot basidiomycetes has several unusual features which are desc ribed below. Feature I: Lignin degradation is part of secondary metabolism: Lignin degradation occurs only after primary growth is complete i.e. during secondary metabolism. Production of ligninolytic enzymes in the cultures of white rot fungi occur when the primary growth ceases in response to limitation 2 of nutrient C / N / SO4 but not PO4. Shift to secondary metabolism occurs to accommodate the limitation (3). Secondary metabolic phase is characterized by the production of secondary metabolites (e.g. antibiotics), a series of developmental changes (sporulation) and demise of the mycelium (decline phase) (4-6). Ligninolytic enzyme system is associated with the secondary and perhaps decline phase. White-rot fungi do not use lignin as a sole source of carbon and energy because lignin degradation does not occur during primary growth. Little lignin carbon enters central metabolic pathway and is evident from the regulatory features of the ligninolytic system that also suggest that fungi do not depend on lignin as an important carbon and energy substrate (6, 7). Feature II: The ligninolytic system is not induced by lignin: Cultures of white rot fungi produce ligninolytic enzymes in response to nutrient C or N or SO4 limitation irrespective of the presence or absence of lignin (6). Ligninolytic activity in the cultures does not increase in the presence of lignin. Ligninolytic system is induced by molecular oxygen. Higher concentrations of molecular oxygen increase the titer and shorten the time of appearance of ligninolytic activity (8-11). Feature III: Lignin polymer degradation involves oxidative reactions: Degradation of lignin polymer initiates by oxidation occurs (8). Presence of molecular oxygen is necessary for the degradation of lignin. A subsequent increas e in the oxygen content and decrease in hydrogen content of partially degraded lignin polymer provides strong proof of the oxidative nature of lignin degradation (12, 13). Introduction of hydroxyl group at C? of the side chains and cleavage of aromatic rin gs bound to lignin explains its degradation as oxidative. Products of lignin degradation are largely watersoluble and carbonyl rich (14). Ligninolytic enzyme system consists of peroxidases, oxidases, reductases, and dehydrogenases and the reactions catalyzed by them are largely oxidative and charge-transfer reactions including reductive. Feature IV: The ligninolytic system is non-specific: Despite the presence of stereo-irregular and heterogeneous structure, lignin is degraded. Ligninolytic system degrades lignins of different compositions i.e. of grasses, 3 hard-woods, soft-woods, etc. The non specificity of system is evidenced from the degradation of modified lignins like Kraft lignin, DHP (polyguaiacol), etc (15). Structurally diverse xenobiotic compounds like, polyaromatic hydrocarbons (PAH), (16-18), nitroaromatics and explosives (19, 20), polychlorinated biphenyls (18, 21), and dyes (22-26) are degraded by the ligninolytic cultures. Feature V: Depolymerization is not an obligatory initial step in lignin degradation: Degradation can also be initiated by modification of lignin polymer (12). Degraded lignins exhibit substantial oxidative changes. C 9subunits in the lignin polymer undergo demeth(ox)ylation, side-chain oxidation and ring cleavage reactions (13, 27). Some of these reactions may cause partial depolymerization. This leads to the generation of low molecular weight lignin degradation products suggesting that all aromatic nuclei are not cleaved while bound in the polymer (28). Thus, lignin degradation can be initiated by any of the two ways; lignin polymer modification and lignin depolymerization. Ligninolytic System: Ligninolytic system is extracellular, highly nonspecific and oxidative in nature. It comprises of enzymatic and non-enzymatic components (Table 1.2.) Table 1.2. Composition of ligninolytic system Enzymatic components Non-Enzymatic components Peroxidases Manganese peroxidase Lignin peroxidase Versatile peroxidase RBBR oxygenase Oxidases Laccase Aryl alcohol oxidase Cellobiose oxidoreductase Dehydrogenases Cellobiose dehydrogenase Pyranose 2-dehydrogenase H2O2-generating oxidases Pyranose 2-oxidase Glyoxal oxidase Aryl alcohol oxidase Reductases Quinone reductases 4 Reactive oxygen species Veratryl alcohol Slime MnII Quinones Organic acids H2O2 Enzymatic components of ligninolytic system Manganese peroxidase: Manganese peroxidase (E.C.1.11.13) was discovered in Phanerochaete chrys osporium (29, 30). It is glycosylated, haem-containing protein with a large isozymes family. The molecular weight is in the range of 42-46 kDa (31). One mole of iron protoporphyrin IX per mole of enzyme is present. The family of isozymes have identical catalytic properties but vary in their kinetic constants, substrate specificities, isoelectric point, carbohydrate content and amino acid composition. The catalytic cycle of manganese peroxidase resembles to that of the classical peroxidase, horseradish peroxidase. In the presence of H2O2, native enzyme is converted to a 2e ¯ deficient compound I. Compound I oxidizes Mn II to MnIII and gets converted to compound II which in turn oxidizes another Mn II to return back to native state (Fig 1.2). The conversion of compound I to compound II is also facilitated by phenolic substrates but the conversion of compound II to native enzyme occurs only in the presence of MnII . FERRIC ENZYME H202 H20 Mn III COMPOUND III COMPOUND I MnII MnII H20 excess H202 MnIII COMPOUND II Figure 1.2 Catalytic cycle of manganese peroxidase Fe IV=O centre is partially buried which is accessible to small compounds like Mn II but not organic substrates. Therefore, the completion of catalytic cycle of manganese peroxidase necessitates the presence of Mn II (32). 5 MnIII alone and chelates of Mn III with organic acids (oxalate, malonate, malate etc.), can diffuse from their site of generation and cause one electron oxidation of various phenolic compounds, amino aromatic compounds and low redox potential non-phenolic compounds via hydrogen abstraction generating corresponding radicals (32). reductant MnP( I / II ) MnII HQ• MnP( II / N ) MnIII H2Q Assist in maintaining H2Q pool for MnP-catalyzed reduction reactions recycling of quinones Substrate Q Substrate reduced Cellobiose dehydrogenase Pyranose-2-dehydrogenase Cellobiose oxidoreductase Quinone reductase Figure 1.3 Reduction reactions catalyzed by manganese peroxidase Manganese peroxidase can also catalyze reduction reactions (Fig 1.3). Reduced quinones (H 2Q) are generated by various dehydrogenases (33) and reductases secreted in the ligninolytic pool of enzymes. Mn III can oxidise these reduced quinones to 1e ¯ oxidised quinones (HQ•). These 1e¯ oxidised quinones are than stabilized by getting further oxidized to native quinones, simultaneously, reducing range of other oxidised chemicals. The radical (oxidants and reductants) generating characteristics of manganese peroxidase underline its potency in bioremediation, to bring about the degradation of xenobionts. Lignin peroxidase : Lignin peroxidase (E.C.1.11.14) (34, 35) is a heme-containing glycoprotein with a molecular weight of 41-42 kDa. It is optimally active, but not stable at low pH (pH 2) (36). Lignin peroxidase is represented by a family of isozymes that vary with strains and cultural conditions (37). 6 The native enzyme reacts with H2O2 to generate compound I, a two electron oxidized intermediate, which is converted to one electron-oxidized com pound II via oxidation of veratryl alcohol (VA) generating VA cation radical VA+•. VA+• can undergo a variety of reactions and can mediate oxidation reactions. Compound II returns to native state by oxidizing another molecule of VA to complete its catalytic cycle (Fig 1.4). In the presence of excess of H2O 2, compound II can be converted to compound III, an oxyperoxide, which is an inactive form of enzyme (38). FERRIC ENZYME VA VA +• H202 H 20 VA+• COMPOUND III COMPOUND I VA VA H20 excess H202 VA +• COMPOUND II Figure 1.4 Catalytic cycle of lignin peroxidase Lignin peroxidase can also catalyze reductions using secondary metabolites that are produced by the fungus (Fig 1.5). The VA+• generated by lignin peroxidase can oxidize oxalic acid producing oxalate anion radical which in turn can reduce variety of chemicals (39). Oxalate and VA act as redox mediators in lignin peroxidase catalyzed reduction reactions. The redox reactions that lignin peroxidases can catalyze stress their potential in bioremediation program. LP( I / II ) LP( II / N ) VA anion radical CO 2 + CO2 ¯ • Substrate CO 2 Substrate reduced Organic acid Figure 1.5 Reduction reactions catalyzed by LP VA+• 7 Versatile peroxidase : Versatile peroxidase, another novel peroxidase, has recently been reported from the cultures of Pleurotus eryngii and Bjerkandera adusta (40, 41). Versatile peroxidase catalyzes the reactions catalyzed by both the ligninolytic fungal peroxidases, namely, manganese peroxidase and lignin peroxidase. It has an ability to oxidize the substrates of manganese peroxidase and lignin peroxidase. It oxidizes Mn II to Mn III and veratryl alcohol to its cation radical. It also oxidizes hydroquinone and substituted phenols, substrates of plant peroxidase and is able to degrade lignin model dimers veratryl glycerol β-guaiacyl ethers (42). Versatile peroxidase, produced by Pleurotus eryngii, can be considered as third type of ligninolytic peroxidase. RBBR Oxygenase: Pleurotus ostreatus during solid state fermentation of wheat straw produced a H2O2-dependent enzymatic activity that decolorized Remazol brilliant blue R (26). This activity is different from manganese peroxidase, laccase, and veratryl alcohol oxidase produced by the fungus and from lignin peroxidase which is not produced by Pleurotus ostreatus but are well known in other white rot fungi. Laccase : Laccase (E.C.1.10.3.2) is a blue copper oxidase. It is a glycoprotein having molecular weight of 60-80 kDa. It is capable of oxidizing electron rich phenolic substrates. Laccase contains multiple copper atoms which are reduced as substrates are oxidized. After four electrons are received, laccase converts molecular oxygen to water (43). Laccase catalyzes one electron abstraction from mono- and poly-phenolics and aromatic amines, to generate free radicals capable of undergoing further depolymerizatioin, repolymerization, demethylation and quinone formation (44). A new laccase, yellow laccase has been discovered recently in the cultures of Panus tigrinus and Phlebia radiata capable of oxidizing non-phenolic lignin m odels and veratryl alcohol in the absence of any mediator (45). Substrate specificities of laccase can be further expanded by adding redox mediator like 2,2’-azinobis(3-ethylbenzothiozoline-6-sulphate) but there is no report whether such 8 mediators function in vivo in lignin degradation, and thus demand further research to elucidate the actual role of laccase (46). Aryl alcohol oxidase: Aryl alcohol oxidase (E.C.1.1.3.7) was first detected by Farmer et.al (1960) (47). Aryl alcohol oxidases are flavo -proteins containing one molecule of FAD per molecule of enzyme. Aryl alcohol oxidase has wide substrate specificity towards differently substituted methoxy phenol. Aryl alcohol oxidase catalyzes the oxidation of aryl α and α−β-unsaturated-γalcohols to their respective aldehydes with simultaneous reduction of O2 to H2O 2. It has been proposed that aryl alcohol oxidase and intracellular NADPH dependent aryl alcohol dehydrogenases could constitute a redox system involving aryl alcohol/ aldehydes produced in order to ensure a steady state concentration of H2O2 for ligninolytic activities (48). Glyoxal oxidase: Glyoxal oxidase (E.C.1.2.3.5) yields three molecules of hydrogen peroxide from glyceraldehydes generated by lignin peroxidase from Cα-Cβ cleavage of aryl glycerol-α -aryl ethers (49). The various substrates for glyoxal oxidase are methyl glyoxal, glycolaldehyde, acetaldehyde, formaldehyde, glyoxal, glyoxalic acid, dihydroxyacetone and glyceraldehydes. Thus, the mutual working of glyoxal oxidase and lignin peroxidase leads to controlled radical formation and limitation of radical coupling, driving lignin oxidation towards depolymerization. Pyranose 2-oxidase: Pyranose 2-oxidase (E.C.1.1.3.10) is homotetrameric protein that contains covalently bonded flavin adenine dinucleotide (FAD) (50). It is localized in periplasmic space, but during autolysis it is localized extracellularly. Pyranose oxidases are widely distributed among wood degrading basidiomycetes and catalyze the conversions of aldopyranoses to corresponding 2-keto aldoses like glucose, galactose and xylose to 2-ketoglucose, 2-ketogalactose and 2-ketoxylose (51). During this conversion, the oxidation reaction, electrons are transferred to molecular oxygen resulting in the formation of hydrogen peroxide; a must needed substrate for ligninolytic peroxidases. 9 Glucose oxidase: Glucose oxidase (E.C.1.1.3.4) while oxidising glucose, generates H2O2 from molecular oxygen (52). It also reduces quinones and phenoxy radicals generated by la ccase during oxidation of lignin (53). The excess of quinones inhibit laccase activity. Thus, glucose oxidase, in turn acts as a regulator of enzymes involved in transformation of lignocelluloses by reducing the quinones and by producing H 2O2. Cellobiose dehydrogenase: Cellobiose dehydrogenase (E.C.1.1.99.18) is a high molecular weight, 90 kDa, flavo -hemo-protein. It is having two prosthetic groups, FAD and heme at two different domains. Heme free portion of cellobiose dehydrogenase was thought to be a separate enzyme, Cellobiose:quinone oxidoreductase. Cellobiose dehydrogenase oxidizes cellobiose to cellobionolactone and prevents the recondensation of the glycosidic bond of the cellulose chain nicked by endoglucanases (54). Cellobiose dehydrogenase reduces quinones to phenols that can be used as redox mediator during ligninolysis and also reduces aromatic radicals, generated as a result of lignin degradation by the ligninolytic enzymes (55). Cellobiose dehydrogenase generates H2O2 and superoxide anion and oxidizes cellobiose to transfer 2e − to cytochrome thus producing ample supply of energy to the fungus. It assists the ligninolytic peroxidases by reducing compound II and especially, manganese peroxidase by dissolving the Mn(IV)O2 precipitates (56). Pyranose 2-dehydrogenase: It is a sugar C2-oxidoreductase having molecular weight of 79 kDa (57). A number of monosaccharides, oligosaccharides and glucosides serve as substrates for Pyranose 2dehydrogenase. It degrades cell wall polysaccharides thus interconnecting lignocellulose decomposition by ligninolysis. This is proposed to reduce quinone thus facilitating the smooth oxidation of lignin (57). 10 Non-enzymatic component of ligninolytic system Veratryl alcohol: Veratryl alcohol, generated as a part of sec ondary metabolism from glucose is a product of lignin degradation. Veratryl alcohol plays a very important role in lignin peroxidase-catalyzed reductive ligninolysis (58, 59). It not only acts as an inducer but also as a substrate. It facilitates the redox reactions, catalyzed by lignin peroxidase, by acting as a redox mediator (59). Though, lignin peroxidase can catalyze some reactions in the absence of veratryl alcohol but veratryl alcohol serves as a mediator for many redox reactions. Veratryl alcohol prevents the formation of compound III and compound IV, an inactivated form of lignin peroxidase due to excess of H2O 2, and can also cause reactivation of these intermediates (38). MnII: MnII acts as a substrate and redox coupler for manganese peroxidase. It also acts as an inducer (controlling at the transcriptional level) for the synthesis of manganese peroxidase in ligninolytic cultures. Manganese peroxidase catalyzed oxidation and reduction reaction demands the Mn II as a mediator (60). H2O 2: H2O2 formed simultaneously with ligninolytic system is an inseparable part of lignin degradation. It serves as a co-substrate of ligninolytic peroxidases. It is also helps in generation of •OH radical, a very potent reactive oxygen species. The reaction by which •OH radical is generated is termed as Fenton’s reaction (61). FeII+ + H2O2?? ?•OH + FeIII+ OH– Thus, H2O2 supports ligninolysis in two major ways: 1) activates peroxidases 2) supports generation of •OH radical. Organic acids: These are synthesized and secreted during secondary metabolism. Organic acids generate microenvironment necessary for 11 ligninolytic peroxidase activity by lowering the pH. It can chelate Mn III and stabilize it which can diffuse away from the mycelial growth and can bring about oxidation of various compounds in the remote sites. Organic acids can serve as precursors for the generation of anion radicals (COO-•) that in turn act as reductants and may also assist in the generation of other radicals like - O2 • and •OH (Fig. 1.6). Fenton reaction anion radical LP( I / II ) VA LP( II / N ) VA+ • • CO2 + CO2¯ O2 Organic CO2 acid O2¯ cation radical (oxidant) • anion radical (reductant) Fe II Fe III H 2O 2 OH ¯ + OH • Hydroxyl radical (oxidant) Figure 1.6 Organic acids as precursor for generation of various radicals Quinones: Quinones are degradation products of lignin. Oxidation of methoxy hydroquinones during lignin degradation followed by auto-oxidation of the resulting methoxy semiquinones results in the formation of super oxide anion radicals that further serve as a member non-enzymatic component of ligninolytic system (62). Excess production of quinones ceases the process of ligninolysis by inactivating the oxidases. Presence of dehydrogenase prevents the excess accumulation of quinones and thus regulates lignin degradation. Slime: Initiation of lignin degradation by white rot fungi results in the accumulation of slime. The slime generates the microenvironment for lignin degradation that is controlled temperature and pH, dissolved oxygen availability, and aqueous environment. It also serves to concentrate the nutrients for growth and the enzymatic and non-enzymatic ligninolytic components essential for ligninolysis. 12 Importance of lignin degradation by lignin degrading fungi Lignin degradation helps to understand the system of lignin degrading fungi. Since, Lignin degrading fungi play a vital role in catalyzing lignin degradation, the rate-limiting step of the ecologically important earth’s Carbon-cycle. Lignin is a physical barrier in lingo-cellulose decomposition. The breakdown of lignin will therefore help in the bioconversion of lignocellulosic in many ways; first, production of a ruminant animal feed, where breakdown of lignin will simplify the lignocellulosic feed and thus, will increase the access of hydrolytic enzymes such as cellulases and hemicellulases to their substrates (63). This will help in feed production by controlled cultivation of ligninolytic fungi on lignocellulosic substrates. Second, manufacture of mechanical pulp, where simplification of lignocelluloses will act as a process of pretreatment for pulping . This has led to concept of biopulping. Mechanical and chemical processes employed in pulping lead to pollution and demands high energy. Thus, pretreatment could reduce the energy demand for mechanical pulping. Third, white rot fungi are efficient producers of cellulases, hemicellulases and ligninases and can be used in the treatment of waste liquors generated by sulphate pulping (64); for converting lignocellulose wastes to protein rich feed (65) and effluent decolorization of kraft pulp mills (66). Apart from this, lignin degrading fungi can be used for producing commercially important fungal metabolites or lignin-derived chemicals. Solubilized lignin can be used in adhesive formulation, as an immunoadjuvant (67) etc. Fruiting bodies of many lignin degrad ing fungi are edible and can be directly used as a food for humans. Since, they can grow on cheap lignocellulosic waste, generation of edible lignin degrading fungi will not only supplement the human food supply but also the decomposed waste which can be u sed for ruminant animal feed. Thus in brief lignin degrading fungi represent a potentially important group of organisms having a wide range of biotechnological applications (Figure 1.7). The understanding of biochemistry and enzymolozy of lignin 13 degrading fungi has opened a new door for research in the field of bioremediation. Whole cell cultures as well as crude and pure enzymes have been shown to degrade wide variety of xenobiotics compounds and appear to be potent bioremediation tools. Ecologically important rate-limiting step of C cycle Treatment of lignin-containing industrial wastes Treatment of industrial wastes Biodelignification Production of Biomass Lignin biodegradation Production of modified lignin polymers Production of microbial metabolites Production of chemicals from lignin Figure 1.7 Significance of lignin biodegradation 14 CHAPTER 1b WHITE-R OT BASIDIOMYCETES: D EGRADATION OF D YES P ersistence of many organic molecules synthesized by man in the environment provided the impetus for studies on the metabolism of xenobiotics. Compounding the problem of recalcitrance was the bio-concentration and subsequent bio-magnification of many xenobiotic compounds. The ability of microorganisms to metabolize xenobiotic compounds has received much attention due to the environmental persistence and toxicity of these chemicals. Recent advances on the bioremediation of environmentally relevant chemicals has centered on four important aspects: first, the characterization of the biodegradation process useful for the treatment of the xenobiotic compounds; second, the development of technical protocols for increasing the degradation rates and substrate ranges of microbial enzymes; third, the design and engineering of bioreactor systems and bio-treatment strategies to optimize biodegradation process; and fourth, the development of data-base of the ecological and human health risks associated with exposure to the chemicals (68). The recognition that environmental pollution is a worldwide threat to public health has given rise to a new, massive industry for environmental restoration. For both economic and ecological reasons, biological degradation has become an increasingly popular alternative for the treatment of hazardous wastes. White -rot basidiomycetes (WRB) comprise the only group of organisms known to degrade wood completely and employ unique biodegradation mechanisms which confer them with the ability to degrade a wide variety of xenobionts (37, 69). 15 Lignin Degradation by White-rot Basidiomycetes In nature, the white -rot basidiomycetes (WRB) play a significant role in the recycling of lignin -containing plant tissues. They have evolved strategies for breaking down the irregular aromatic structure of the lignin polymer to access and utilize cellulose and hemi-cellulose as carbon sources. Although the lignin degrading ability has been recognized for many years, investigators have now begun to understand the mechanisms by which this degradation is accomplished (70, 71). The lignin degrading enzyme system of WRB is extracellular and unusually nonspecific (37). White -rot fungi utilize simple radical-generating mechanisms for the degradation of lignin. Peroxidases and hydrogen peroxide, which are secreted by WRB, catalyze reactions generating free radicals that cause depolymerization and degradation of lignin. Although lignin is naturally a highly reduced polymer, it is mineralized to carbon dioxide and water by WRB. Understanding how this is accomplished is central to understanding degradation of environmental pollutants by the fungi. The extracellular biodegradation system also explains why the fungi can be quite resistant to toxic or mutagenic chemicals. White -rot Basidiomycetes: Dyes Degradation White-rot basidiomycetes (WRB) are able to degrade pesticides, polyaromatic hydrocarbons, PCBs and other halogenated aromatics (including dioxins), dyes, nitro aromatics, and toxic chemicals such as cyanides, azides, carbon tetrachloride, and pentachlorophenol. WRB are also nonselective in degrading all the chemical components of complex mixtures. For example, all the components of Aroclors, toxaphene, creosote, and coal tars are degraded by the fungi (72, 73). This ability is related to the nonspecific nature of the peroxidases secreted by the fungi and to the variety of mechanisms that the fungi use to degrade chemicals. 16 Synthetic dyes are extensively used in textile, paper, photography, cosmetics and leather industries. Between 2-50% of the total dye consumed in dyeing process are found in waste-waters depending on the class of dye application (74, 75). Dyes possess several structural varieties such as acidic, reactive, basic, disperse, azo, sulfonphthalein, triphenyl methane, anthraquinone-based and metal-complex etc. and thus represent an important role model for studies on xenobionts degradation. Dyes are chemically stable that resist degradation by microbial attack (22) and therefore, persist in conventional biological wastewater treatments. Glenn and Gold (1983) suggested that the decolorization is a secondary metabolic activity linked to the fungus ligninolytic activity (76). Such secondary metabolizing ligninolytic cultures of Phanerochaete chrysosporium decolorize structurally diverse polymeric dyes (Poly B-411, Poly R-481, and Poly Y-606), crystal violet, para-rosaniline, cresol red, bromophenol blue, ethyl violet, malachite green, brilliant green and azo and heterocyclic dyes (77, 78). Thereafter, degradation of azo, anthraquinone, heterocyclic, triphenyl methane and polymeric dyes by WRB has been intensively studied (73, 79, 80). Degradation of copper-phthalocyanine dyes by P. chrysosporium resulted in copper and organo copper breakdown products, which were found in culture supernatants (81). Jarosz-Wilkolazka et al. screened 115 fungi of different physio-ecological group for their ability to decolorize azo and heterocyclic dyes, concluding h t at heterocyclic dyes are decolorized easier and faster by fungi than azo dyes (8 2). Recent investigation has shown the involvement of Thelephora sp. in the decolorization of azo dyes and dye industry effluents (83). This emphasizes the need of large-scale screening program for WRB to identify potent species on behalf of their ability to degrade dyes. Dye decolorization can be used as an alternative tool to radiolabeled lignins for lignin degradation bioremediation for other xenobionts. 17 studies and in programming Involvement of lignin-degrading enzymes in the decolorization of dyes was investigated by the several workers (79, 84 ). The first report of aerobic degradation of azo dyes by white-rot fungi appeared in 1990, when Cripps et al reported decolorization of azo dye Acid Orange 7 by nitrogen limited cultures of P. chrysosporium (78). Decolorization of methylene blue by lignin peroxidase (LIP) of P. chrysosporium provided evidence the oxidative mode of degradation (80). Spadaro et al reported mineralization of azo dyes by the ligninolytic enzymes (85). Two groups reported degradation of different sulfonated azo dyes by crude and purified peroxidase preparations, respectively (73, 86). LIP activity of P. chrysosporium was analyzed for the decolorization of 50 structurally different dyes (87). Oxidation of nonsulfonated azo dyes [1-(4’-acetamidophenylazo)-2-napthol] by LIP from P. chrysosporium resulted in the formation of 1,2-napthaquinone and acetanilide (88). It was believed that manganese peroxidase (MnP) and laccase convert a limited variety of azo dyes and preferred dyes carrying a phenolic substituent in para- position to the azo bond and additionally methyl- or methoxysubstituents in 2- or 2, 6- position in relation to hydroxyl- group (89). Later it was shown that the MnP from of Bjerkandera adusta and Pleurotus eryngii catalyses decolorization of structurally diverse dyes in a Mn 2+-independent reaction (90). Recently Moreira et al. demonstrated decolorization of Poly R478 using MnP (91). Laccases from Pycnoporus cinnabarinus is able to decolorize complex, industrially relevant azo dyes such as Reactive Blue 5 and Direct Blue 1 (92). Our group reported the decolorization of sulfonphthalein dyes by ligninolytic enzymes of different WRB (23, 25). Vyas and Molitoris reported decolorization of RBBR by a novel enzyme, RBBR oxygenase, produced by Pl. ostreatus , which is different from MnP, LIP, veratryl alcohol oxidase and laccase (26). They reported decolorization of RBBR by WRB that undergoes sequential change of blue dye to colorless through a rainbow of intermediates also shown by other researchers (93). Such observations strengthen the fact that decolorization is not a single step reaction and intermediates are involved during complete degradation. Decolorization of polymeric dyes Poly R-478 and Poly S-119 have been shown by the cultures of P. chrysosporium in the static conditions as 18 well as immobilized in a bioreactor (94). Palma et al. correlated the decolorization of the anthraquinone dye Poly R-478 with the ligninolytic activity of an immobilized P. chrysosporium in a continuous -flow pulsing packed bed reactor (95). Involvement of degradative mechanism other than ligninolytic enzyme for dye decolorization has also been studied. Plasma membrane dependent redox system, of the fungus, allows adjusting the pH of its nearby surrounding environment and maintains proton gradient across the plasma membrane. Pasti & Crawford proposed plasma membrane redox system of WRB for dye decolorization (96). Though dye decolorization has not been studied by this system still the report on the mineralization of TNT by cultures of P. chrysosporium explains the mechanism employed (19). The investigation reports the reduction of TNT to mono- and di-amino congeners by transmembrane redox potential, which are further oxidized by MnP and subsequent evolution of CO2 is associated with the production of LIP. Report on the new enzymes, RBBR oxygenase and versatile peroxidase, of ligninolytic system and other redox system involved in degradation and the inherent complexity of dye molecules makes it difficult to identify the degradative pathways utilized by WRB. The promising challenge is to synchronize the parameters, like, environmental and nutrients, for whole cell cultures and to integrate the role of various enzymes and other redox machineries to come up with a total picture of how xenobionts are degraded by white -rot basidiomycetes. White -rot basidiomycetes: Mechanisms of Dye Degradation Conventionally, coagulation and flocculation using Fe, Al, and Mg salts have been practiced for the decolorization of dye wastewaters. The problems associated with such process es are (i) limitation of chemical coagulation– flocculation to produce good quality effluent, (ii) sludge handlin g problems, (iii) the technical and economical constraints of reverse osmosis due to short 19 membrane life, (iv) economical non-feasibility, and (v) possible detrimental effects of chemical oxidation using oxidative agents (22, 97, 98). White-rot basidio mycetes degrade PAH, PCB, benzo[α ]-pyrene and benzo[π]dioxins (18, 21, 99, 100). Initial reports not only established the nonspecificity of the fungus but also showed its ability to oxidize highly chlorinated chemicals. Such highly chlorinated chemicals are electron deficient due to the high electro negativity of the chlorine. These chemicals must therefore be reduced before they can be oxidized (101). White rot fungi synthesize and secrete a substrate for its own peroxidase: veratryl alcohol (3,4-dimethoxybenzyl alcohol). The partially oxidized veratryl alcohol (the cation free radical) then oxidizes other chemicals that are not directly oxidized by the peroxidases (58, 102). To accomplish further degradation, the fungus produces organic acids such as, oxalates that also inhibit veratryl alcohol oxidation (103). Why would fungus produce a substrate and an inhibitor of its own enzymes? Oxalate is easily oxidized to carbon dioxide, thus its oxidation is essentially irreversible. However, the oxidation of oxalate is a two-electron oxidation, whereas the reduction of the veratryl alcohol cation radical consumes only one electron (104). Therefore, the odd electron left in oxalate is available for other reductions. A number of electron acceptors can thus be reduced by LIP provided with hydrogen peroxide, veratryl alcohol (which is now called a "mediator"), and oxalate (which has been termed the "donor"). Though the degradation of dye has not been studied by this system yet the reductive dechlorination of carbon tetrachloride (a highly oxidized chemical) to the trichloromethyl radical was demonstrated with this system (105). The reductive dechlorination process accomplished using a peroxidase, thus opens up a new field of investigation into the role of peroxidases in the degradation of dyes requiring reduction. Molecular oxygen can also be reduced by the oxalate radical to give superoxide, a species of oxygen that is useful for either oxidation or reduction. At low pH, superoxide is an excellent oxidant. Alternatively, superoxide is a 20 good reductant, especially at higher pH, for reductive dechlorinations (106). Superoxide is also useful to generate another powerful oxidant, the hydroxyl radical. In the presence of transition metals, such as iron, superoxide can catalyze the generation of hydroxyl radicals by a sequence of reactions called the Haber-Weiss reaction (107). White-rot basidiomycetes produce hydrogen peroxide which can react with Fe 2+, termed as Fenton's reagent (108), to produce hydroxyl radical. WRB produces oxidants, like superoxide, hydroxyl radical etc., and reductants, like oxalate-VA system etc., which generates redox-pump. It can prove to be one of the important machinery in degradation of dyes. Interest in the pollution potential of synthetic dyes has primarily been prompted by concern over their possible toxicity and carcinogenicity. Many dyes are made from known carcinogens, such as benzidine and other aromatic compounds, all of which might be reformed as a result of physicochemical transformation (109). It has been shown that azo dyes are reduced in sediments resulting in the parent toxic amines (110). Eaton et al. reported for the first time biological decolorization of lignin -containing waste-water from pulp and paper industries by two white-rot basidiomycetes, P. chrysosporium and Tinctoporia sp (111). The activities of both the fungi were clear examples of color removal through microbial degradation of polymeric lignin molecules. Since then, P. chrysosporium in particular has been the subject of intensive research related to the degradation of a wide range of synthetic dyes. Table1.1b summarizes some of the reports on dye decolorization by the white-rot fungi. Phanerochaete chrysosporium decolorizes azo and heterocyclic dyes, such as Orange II, Tropaeolin O, Congo red, and Azure B (78). The extent of color removal varies depending on the dye complexity, nitrogen availability in media, and the ligninolytic activity of the culture. At low nitrogen concentrations, 90% of the color was removed within the initial 6 h; however, in excess nitrogen concentrations, 63–93% decolorization of the above mentioned dyes took up to 5 days (78). For P. chrysosporium, acidic conditions (pH 3.5–5) and concentration of VA > 1 mM and hydrogen peroxide (of 0.2 or 0.8 mM, as per the dye structu re) are highly favorable (113). 21 Azo dyes are the most widely studied dyes for decolorization studies using white-rot fungi. Decolorization of azo dyes by ligninolytic enzymes is an oxidative process that can result in complete degradation of the dye mole cule to CO 2 and H2O (85, 122). The presence of the secondary metabolite VA stimulates the decolorization by the fungi, by inducing the LIP activity (79, 84, 123). LIP oxidizes azo dyes where VA serves as a redox mediator. (84). Indigo carmine, an azo dye, is a good substrate of MnP and LIP, and it has been reported that although the dye is successfully decolorized by both groups of extracellular peroxidases, the reaction by MnP is faster (87). Although no general pattern has been established between the structure of dyes and decolorization, it can be inferred from the research so far that the dyes that are structurally similar to the natural substrates will be decolorized faster and more efficiently. Dyes with structural similarities may still be differentially decolorized by the white-rot fungi, for the reason that electron distribution and charge density along with stearic distribution may differ (124). Decolorization of Poly R-478 was used by Freitage and Morrel for screening 170 strains of white-, brown-, and soft-rot fungi, as well as xylophilous fungi for their peroxidase and phenol oxidase activity (125). They concluded that no correlation exists between the MnP or LIP and decolorization of dyes. Heinfling et al. initially showed the decolorization of phthalocyanines dyes Reactive blue 38 and Reactive blue 15 by B. adusta, P. chrysosporium, and Trametes versicolor (119). Later they reported the role of MnP isozymes involed in the decolorization of these dyes (120). The ability of the WRB to degrade the synthetic dyes has been exploited for the potential use of dyes as substrates for enzyme activity. Pasti-Grigsby et al. examined a number of azo dyes and found that Orange I and 3,5-dimethyl-4hydroxyazobenzene-4'-sulfonic acid served as good substrates for MnP, while Orange II and 3,5-difluoro -4-hydroxybenzene-4'-sulfonic acid were better for LIP (126). Transformation and degradation of the disazo dye Chicago Sky Blue by a purified laccase from Py . cinnabarinus opened the possible use of laccase in the dye degradation studies (74). A Brazilian strain of Pl. pulmonarius produces only laccase and no MnP, LIP, or aryl alcohol oxidase, decolorizes dyes of different spectra (127). 22 Table 1.1b Dyes decolorized by some known white-rot basidiomycetes Organism Dye Phanerochaete Bromophenol blue, Congo red, Methyl Green, Methyl chrysosporium Orange, Poly R-478, RBBR, Toluidine Blue O, Poly S-119, Poly T-128 Orange II Methyle ne Blue Crystal violet Disperse Orange 3 Disperse Yellow 3 Indigo carmine, Cibacron Brilliant Red, Brilliant green Amaranth, Remazol Black B, Remazol Orange, RBBR, Reactive Blue, Tropaeolin O Polymeric Dyes, B-411, R-481, Y-606 Acid Yellow 9 Trametes versicolor Remazol Black B, Remazol Orange, RBBR, Reactive blue Indigo carmine, Reactive blue 15, Acid Blue 25, Acid Black 24 Crystal violet Pigment Violet 12 Orange II Reactive orange 96, Reactive violet 5, Reactive black 5, Reactive blue 38 Bjerkandera adusta Reactive blue 38, Reactive violet 5, Reactive black 5, Reactive orange 96, Reactive red 198, Reactive blue 15 Irpex lacteus RBBR Bromophenol blue, Cu-phtha locyanine, Methyl Red, Congo red Pleurotus ostreatus Acid Black 194, Acid Blue 185, Disperse Blue 56, Sulfur Black 1, Vat Blue 6, Orisol Blue BH, Disperse Yellow 54, Reactive blue 19, Reactive blue 158, Vat Red 10, Vat Yellow 46 RBBR Bromophenol red, Phenol red, Bromophenol blue, mCresol purple, o-Cresol red, Bromocresol purple, Bromocresol green Reference 79 78 80 73 85 88 126 112 76 124 112 113 115 114 116 117 118 119 120 121 26 25 Soares et al. have reported decolorization of Remazol Brilliant Blue R using a combination of redox mediator, laccase, and nonionic surfactant 23 (128). They postulate the necessity of a redox mediator for laccase-catalyzed decolorization of dyes. Summing up the mechanisms used by WRB in degradation of dyes and other xenobionts, explains that enzymatic system together with redoxpumps provide powerful means for the degradation dyes. The varied combination of the above mentioned system and followed by standardized parameters (environmental and nutrient) for the cultivation of fungi is most important to scale-up the results from laboratory to field level. Future work needs to be focused on this area for a sustainable technology using white-rot basidiomycetes. White -rot basidiomycetes: A Promising Candidate for Xenobionts Degradation Biotechnological applications of white-rot basidiomycetes (WRB) and their enzymes are being developed, as alternative methods for pulping and bleaching and for removal of toxic pollutants from soil and water reservoirs. White-rot basidiomycetes have developed unique mechanisms for degradation of recalcitrant compounds such as lignin. It was not until the latter part of the 1980s that the white-rot fungi's capability for degradation was considered also for organo-pollutants. The first studies with WRB, P. chrysosporium and P. sordida in soil were published in 1989-90. Even so P. chrysosporium in liquid culture continued to be the main focus for several years. Since soil is not the natural habitat for WRB, soil remediation requires knowledge from several areas; physiology of WRB, soil chemistry, and soil biology. Future work directed towards identifying novel oxido-reductases that may have auxiliary roles in transformation of dyes that are more recalcitrant could lead to the development of a more effective enzymatic cocktail for dye decolorization. The use of whole fungi, rather than enzymes might be expected to be a more viable option from the point of view of the decolorization process control at the industrial scale for the reason that 24 degradation process is not achieved by a single enzyme but with many enzymes and the varied composition of these enzymes. Again, the degradation is not related only to the enzymatic system but also to the nonenzymatic components that adds up to the degradation by the generation of redox pump and also provides the needed microenviro nment for the degradation process. Thus instead of acclimatizing the system with such factors it become obvious to understand that the whole cell appears to be a more viable option. The failure of success is probably due to an engineering problem rather than a fundamental limitation of the technology itself. Advantage of using WRB is that they do not require preconditioning to particular pollutants and can degrade xenobiotics present in a very low concentration to non-detectable level and even complete elimination. Thus in brief lignin degrading fungi represent a potentially important group of organisms having wide range of biotechnological applications . 25 CHAPTER 2 SCREENING OF WHITE-R OT BASIDIOMYCETES: DECOLORIZATION OF TEXTILE D YES 2.1 Introduction W hite rot basidiomycetes (WRB) are the only known organisms to mineralize lignin, a recalcitrant biopolymer (2). The ligninolytic system of WRB is (I) extracellular (II) non-specific, as they are able to degrade lignins of different origins and composition (15), (III) highly oxidative (37), and (IV) non inducible, as lignin degradation is an idiophasic event (6-7). These properties allow the WRB to degrade a wide variety of xenobionts. Xenobionts are defined as the organic compounds that are foreign to life in general. Such compounds are usually recalcitrant as the cells are never exposed to these chemicals; as a consequence they resist biodegradation and persist in nature. Dyes can be considered as role models of xenobionts because of their; (I) structural diversity, (II) stability, and (III) complexity in structure, which make them resemble diverse groups of xenobionts. It is easy to follow their degradation because of their chromogenic nature. Conventional mechanisms like, photocatalysis, ozonation etc. are not efficient to remove recalcitrant dyes from environment (97,110,111). The environment-friendly and economic option to known conventional systems is biodegradation. Xenobionts that are structurally similar to lignin sub-structures and the mode of action of ligninolytic system of WRB make them potential candidates and provide an efficient alternative in the bioremediation program. Several methods for screening of white -rot basidiomycetes have been reported where dyes are used as substrates (26, 76). We report the 26 decolorization of 7 textile dyes by the white-rot basidiomycetes, Irpex lacteus and strain F (collected from Kathiyawad peninsula). 2.2 Materials and Methods 2.2.1. Microorganisms: The ligninolytic fungus Irpex a l cteus Fr. 238 617/93, originated from Culture Collection of Basidiomycetes Prague (kindly gifted by Šašek, V.), and strain F were used. The strain F was cultivated from the fruit-body collected from Kathiyawad peninsula and isolated in pure in our laboratory. The strains were maintained on malt extract agar plates at 5ºC. 2.2.2. Isolation of Strain F: Lignin degrading white rot fungus F was collected from Tamarindus indicus plant root from Abhapara, Barda Hills. A piece of collected sample was surface ste rilized with 0.1% HgCl2, followed by ethanol washing. In between this procedure sterile double distilled water wash was given regularly. This piece was then placed on water agar and observed for development of mycelia on plate. Mycelia were then transferred to fortified malt extract agar (FMEA) plates. The culture was obtained in pure by making serial transfers and maintained on FMEA at 4ºC. 2.2.3. Textile dyes: Remazol Turquoise Blue G (RTBG), Reactive Orange 16 (RO16), Remazol Yellow FG (RYFG), Remazol magenta HB (RMHB), Reactive blue 21 (RB21), Remazol red H8B (RRH8B), Reactive Red 6BX (RR6BX), Reactive orange 13 (RO13), Reactive brown 18 (RB18), Reactive black 5 (R B5), Reactive golden HR (RGHR) and Remazol Brilliant blue R (RBBR) were used for the decolorization studies. 2.2.4. Decolorization of textile dyes on solid media: The fungi were screened for their ability to decolorize textile dyes using malt extract agar medium (malt extract 30 g.l-1, mycological peptone 5 g.l-1 and agar 15 g.l-1) containing the dyes (100 ppm). The plates were inoculated with a disk (8 mm) punched from the cultures grown on malt extract agar plates and incubated at 28ºC. The plates were observed daily till day 9 for the zone of growth (mm) and the zone of decolorization (mm) of the dyes. 27 2.3 Results and Discussion Irpex lacteus and strain F were screened for their ability to decolorize textile dyes on malt agar medium. The growth of the fungus on malt agar medium with or without dye occurred equally well. None of the textile dyes appeared to inhibit the fungal growth. Dye decolorization on solid media was under older mycelia which always started from the point of inoculation and the zone of decolorization followed the zone of growth. It indicates that the process of decolorization which is associated with older mycelia is perhaps a part of secondary metabolic event when the ligninolytic enzymes are secreted that have been shown to degrade a variety of organo-pollutants. Mycelial growth of strain F covered the entire plate by day 5. Strain F growing on malt agar medium decolorized all the dyes. The decolorization of dyes that initiated after day 2 continues till the dye in the entire plate is decolorized, but decolorization of Reactive golden HR (RGHR) occurred only till day 5. Thereafter no further decolorization occurs as observed from the zone of decolorization. Decolorization of Remazol Turquoise Blue G (RTBG), Reactive Orange 16 (RO3R), Remazol Yellow FG (RYFG) and Remazol Brilliant Blue R (RRBR) was completed in betw een day 6 and 7 (Fig. 2.1). Textile dyes, Reactive Brown 18 (RB18), Remazol Red H8B (RRH8B), Reactive Orange 13 (ROH2R), Remazol Magenta HB (RMHB), Reactive Blue 21 (RB21) and Reactive Red 6BX (RR6BX) were decolorized completely on day 9 (Fig. 2.1). Both the red dyes, viz. RRH8B and RR6BX, were decolorized but the decolorization of RRH8B was faster and was completed on day 8. Similar results were obtained for the orange dyes, viz. RO13 and RO16, and blue dyes, viz. RBBR and RB21, where decolorization of orange dye, RO16 and blue dye, RBBR was completed on day 6. 28 100 100 (b ) (a) 75 75 50 50 25 25 0 0 100 100 (d) (c) 75 50 50 25 25 0 0 100 100 (e) 75 Size of zone (mm) 75 50 50 25 25 0 0 100 100 (h) (g) 75 75 50 50 25 25 0 0 100 100 (j) (i) 75 75 50 50 25 25 0 0 100 100 (k) 75 (f) 75 (l) 75 50 50 25 25 0 0 0 3 6 9 0 3 6 9 Incubation time (days) Figure 2.1 Decolorization and the growth of the white rot basidiomycete Strain F on malt agar medium containing textile dyes (a) RTBG; (b) RYFG; (c) RR6BX; (d) RRH8B; (e) RO13; (f) RO16; (g) RMHB; (h) RB5; (i) RB21; (j) RBBR; (k) RGHR; (l) RB18 29 Growth of I. lacteus and associated decolorization of textile dyes was faster when compared to the strain F. Irpex lacteus decolorized all the textile dyes, under investigation. Decolorization initiated after day 2 and most of the dyes were decolorized by day 5 when the mycelial growth covered the entire plate (Fig. 2.2). Decolorization of textile dyes, RGHR and RYFG, initiated on day 2, like other dyes, were decolorized only till day 5. There was no further increase in the size of zone of decolorization. Decolorization of RTBG, RR6BX, RRH8B, RO13, RB21, and RB18 followed the growth and were decolorized completely on day 9. The decolorization of red dyes (RRH8B and RR6BX) orange dyes (RO13 and RO16) and blue dyes (RBBR and RB21) were varied (Fig. 2.2) and resembled the decolorization process observed in the case of strain F. It is interesting to note that decolorization of blue and black dyes, by both the fungi, initiated by change in colors having absorbance towards lower wavelength. The color of the dyes changed from blue/black to red/orange and than to yellow before getting colorless (Plate 2.1). The same phenomenon of hypsochromic shift has been reported in the in vitro degradation of dyes (26). This clarifies that the degradation of dyes is not a single step reaction but involves formation of several intermediates. (a) (b) (c) Plate 2.1 Decolorization of Reactive black 5 by Irpex lacteus , showing hypsochromic shift; (a) medium with dye; (b) I. lacteus growing on malt extract medium; (c) I. lacteus growing on medium containing dye 30 100 100 75 50 50 25 25 0 0 100 100 (c) 75 Size of zone (mm) 75 (a) (d) 75 50 50 25 25 0 0 100 100 (e) 75 (b) (f) 75 50 50 25 25 0 0 100 100 (g) 75 50 50 25 25 0 0 100 100 (i) 75 (h) 75 (j) 75 50 50 25 25 0 0 100 100 (k) 75 (l) 75 50 50 25 25 0 0 0 3 6 9 0 3 6 9 Incubation time (days) Figure 2.2 Decolorization and the growth of the white rot basidiomycete Irpex lacteus on malt agar medium containing textile dyes (a) RTBG; (b) RYFG; (c) RR6BX; (d) RRH8B; (e) RO13; (f) RO16; (g) RMHB; (h) RB5; (i) RB21; (j) RBBR; (k) RGHR; (l) RB18 31 The same color dyes (red, orange and blue) were decolorized variably by both the basidiomycetes. It has been reported that the dyes that are structurally similar may be differentially decolorized by white rot fungi. The reason may be attributed to the differences in the electron distribution and charge density along with the steric -distribution in different dyes (126). Growth of both the fungi was similar on the malt agar plates; the growth was slower till day 2 and thereafter increased sharply. The plates were entirely covered by day 5. Both the fungi decolorized all the textile dyes investigated. Irpex lacteus decolorized textile dyes faster as compared to strain F. The present work provided important lead regarding the ability of the white rot fungus to produce some of the ligninolytic activities that need to be purified and characterized. The wood-rotting white rot basidiomycete, I. lacteus is a better alternative than strain F in the degradation study concerning bioremediation program. The ability of I. lacteus to decolorize various groups of dye and the correlation between dye decolorization and ligninolytic activities should be addressed. Such work would allow comparison between the ligninolytic activities of this fungus with others of its kind. 32 CHAPTER 3 LIGNINOLYTIC ENZYMES OF IRPEX LACTEUS: D ECOLORIZATION OF TEXTILE A ND TRIPHENYL METHANE D YES 3.1 Introduction D yes are tailor-made for their stability. It is known that 90% of reactive textile dyes entering activated sludge sewage treatment plants pass through unchanged and are discharged to rivers (129). Not all dyes currently used could be degraded and/or removed with physical and chemical processes, and sometimes the degradation products are more toxic (130). A great variety of synthetic dyes are used for textile dyeing and other industrial applications. The structural diversity of dyes derives from the use of different chromophoric groups (e.g., azo, anthraquinone, triphenyl methane, and phthalocyanine groups) and different application technologies (e.g., reactive,direct, disperse, and vat dyeing) (74). Lignin-degrading white rot fungi have received worldwide attention for their industrial use in bio -pulping, bio-bleaching, dye decolorization, and in detoxifying recalcitrant environmental pollutants (16, 85, 131-133). The ligninolytic system of these fungi is extracellular, non-specific and noninducible, which allows the fungi to mineralize lignin and variety of organic chemicals. Most information on these fungi comes from studies with Phanerochaete chrysosporium and few other species, thus representing only a small part of many hundreds of species existing in nature. Irpex lacteus is a cosmopolitan white rot basidiomycete typically inhabiting dead hard wood trees (134). But, its role in degradation of xenobionts is largely unknown; except for the report on transformation of pentachlorophenol from soil (135). In our report, we investigated decolorization of textile dyes and triphenyl methane dyes by the ligninolytic activities of Irpex lacteus. 33 3.2 Materials and Methods 3.2.1. Microorganism: Irpex lacte us Fr. 238 617/93 obtained fro m Culture Collection of Basidiomycetes (CCBAS), Prague (Kind gift from V. Šašek). The strain is maintained on malt extract agar plates at 4 ºC. 3.2.2. Textile Dyes : Reactive black 5, Remazol black HR , Reactive orange 13, Reactive orange 16, Remazol red H8B, Remazol magenta HB, Remazol turquoise blue G, Remazol lemon yellow F4, Remazol golden yellow R, Reactive brown 18 and Remazol brilliant blue R were obtained from the dye manufacturing and textile processing industries. 3.2.3. Triphenyl methane Dyes: Brilliant green, Methyl blue and Fast green were procured from Hi-Media (India). 3.2.4. Production of ligninolytic enzymes by I. lacteus : Solid state fermentation of wheat straw by I. lacteus was performed as described previously [1 36]. To the culture flasks, harvested on 20th day, 100 ml of phosphate buffer (0.1 M, pH 6.5) containing 0.1 M NaCl was added, contents were gently beaten and incubated on rotary shaker for 1 h. Liquor obtained was then filtered and spun (6000 rpm, 15 min, 5oC) before being subjected to ammonium sulfate precipitation (80% saturation). Precipitates separated upon centrifugation (6000 rpm, 15 min, 5 oC) were dissolved in the minimum amount of phosphate buffer (0.1 M, pH 6.5). Enzyme extract thus obtained was desalted using PD10 column (Pharmacia) and stored in small aliquots at -5ºC for further biochemical analysis. 3.2.5. Enzyme assays: Manganese peroxidase (MnP), manganeseindependent peroxidase (MIP) and lignin peroxidase (LIP) activities were analyzed using dyes as the substrate. Reaction mixture (2 ml) for MnP activity contained 100 µmol sodium tartarate buffer (pH 2.5, 3.0 and 3.5), succinate lactate buffer (pH 4.0 and 4.5) and sodium citrate buffer (pH 5.0 and 5.5), 0.2 µmol MnSO4, 150 ppm textile dyes or 75 ppm triphenyl methane dyes, 0.4 µmol H2O2 and the enzyme extract. Reaction mixture for MIP activity was similar to that of MnP but MnSO4 was not added. Reaction mixture for LIP 34 activity was similar to that of MnP but veratryl alcohol (4 µmol) was added instead of MnSO 4. One unit enzyme activity is defined as the amount of activity that consumes 1 µmol of the triphenyl methane dye or produces a change of one absorbance unit per min upon oxidation of textile dye at its λmax. 3.3 Results and Discussion Irpex lacteus , during solid state fermentation of wheat straw, produced ligninolytic activities, namely, manganese peroxidase (MnP), manganeseindependent peroxidase (MIP) and lignin peroxidase (LIP). Decolorization of 11 textile dyes and 3 triphenyl methane dyes by the ligninolytic enzymes produced by I. lacteus was investigated at varying pH (Fig 3.1 and 3.2). All the dyes investigated were decolorized by one or the other ligninolytic activities except remazol golden yellow R (RGYR) and brilliant green (BG). 3.3.1. Decolorization of textile dyes by MnP activities: MnP decolorized two textile dyes, reactive orange 16 (RO16) and remazol magenta HB (RMHB) (Fig 3.1). Dyes were decolorized over a broad pH range (2.5 -5.5) and were best decolorized at the MnP pH optimum (4.5). Decolorization of textile dyes increased rapidly above pH 3.0. Decolorization of RO16 occurred in the pH range of 2.5 to 5.0 and RMHB in the pH range of 2.5 to 5.5. Decolorization of RO16 declined sharply above pH 4.5. Dyes that contain free phenolic moiety are better substrates of MnP as MnP prefers aromatic compounds having free phenolic moiety (25, 32). 3.3.2. Decolorization of triphenyl methane dyes by MnP activities: Ligninolytic enzymes catalyze decolorization of synthetic dyes (2 4-26, 137). Decolorization of triphenyl methane dyes was only associated to the MnP activities (Fig 3.2). MnP decolorized methyl blue (MB) and fast green (FG) but was unable to decolorize brilliant green (BG). 35 30 20 20 10 10 0 0 45 30 30 15 15 0 0 RO13 30 % Decolorization RBBR 40 20 0 21 BHR 45 RO16 60 % Decolorization 30 RBB RTBG 20 10 0 60 RRH8B 14 40 7 20 0 0 21 15 LYF4 RMHB GYR 14 10 7 5 0 0 2 15 3 4 5 6 pH BrH4R 10 5 0 2 3 pH 4 5 6 Figure 3.1 Decolorization of textile dyes by manganese peroxidase (?), manganese-independent peroxidase (? ), and lignin peroxidase (? ): (a) Reactive black 5; (b) Remazol black HR; (c) Reactive orange16; (d) Reactive orange 13; (e) Remazol brilliant blue R; (f) Remazol turquoise blue G; (g) Remazol red H8B; (h) Remazol magenta HB; (i) Remazol lemon yellow F4; (j) Remazol golden yellow R; (k) Reactive brown 18 36 Decolorization of RMHB, MB and FG, was associated only with the MnP activity. This signifies that certain dyes are better substrates of MnP and therefore can be employed to differentiate different ligninolytic activities and for studying MnP catalyzed reactions. Dye decolorization has been used as an indicator and measure of ligninolytic activity (35, 138). 3.3.3. Decolorization of textile dyes by LIP activity: Decolorization of RO16 and reactive brown 18 (RB18) was associated also with the LIP activity (Fig 3.1). Decolorization of RO16 occurred over a pH range of 2.5 to 5 but was best at pH 2.5, the pH optimum of LIP. Further increase in the pH from 2.5 led to the slower decolorization. Reactive brown 18 was decolorized in the pH range of 2.5 to 4. Decolorization of RB18 increased sharply above pH 2.5 and was best decolorized at pH 3.5. Above pH 3.5 the decolorization declined. 15 BG 10 5 % Decolorization 0 45 MB 30 15 0 45 FG 30 15 0 2 3 4 5 6 pH Figure 3.2 Decolorization of triphenyl methane by manganese peroxidase (?), manganese-independent peroxidase (? ), and lignin peroxidase (? ): (a) Brilliant green; (b) Methyl blue; (c) Fast green. 37 3.3.4. Decolorization of textile dyes by MIP activity: Decolorization of most of the textile dyes was associated with the MIP activity. MIP activity did not decolorize the triphenyl methane dyes. Anthraquinone, azo and phthalocyanine dyes have been reported to be decolorized in a manganeseindependent enzymatic oxidation (26, 40). MIP showed the highest non specificity in selection of textile dyes for decolorization and decolorized textile dyes over a broad pH range (2.5 -5.5) (Fig 3.1). Unlike MnP, the pH optimum for decolorization by MIP activity was dependent on the dyes. Remazol brilliant blue R (RBBR), RB18 and remazol lemon yellow F4 (LYF4), were decolorized best at pH 2.5 and 3.0. Reactive black 5 (RB5), remazol red H8B (RRH8B), reactive orange 13 (RO13), and remazol black HR (BHR) were decolorized best at 3.5, the pH optimum of MIP. Remazol turquoise blue G (RTBG) was decolorized best at pH 4.0. The variation in the pH for the decolorization of dyes by MIP is indicative of pH as the function of enzymecatalyzed dye decolorization. 3.3.5. Influence of pH on the decolorization of textile dyes: pH plays a crucial role in the decolorization of textile and triphenyl methane dyes. Decolorization by the concentrated crude enzyme extracts prepared from I. lacteus-infested wheat straw occurred over a pH range of 2.5 to 5.5 (Fig 3.1 and 3.2). MnP and LIP decolorized the dyes most at their respective optimal pH. Optimal pH for the decolorization associated to MIP activity varied with the dye. Differences in the production of ligninolytic enzymes make WRF to differ in their ability to degrade and decolorize various dyes. As a result of nonspecificity of lignin-degrading enzymes, individual azo-, heterocyclic -, phthalocyanine-, polymeric-, triphenyl methane- and anthraquinonic dyes (120, 137-139) as well as complex industrial effluents are efficiently decolorized by these fungi (140-140). 3.3.6. Structure function relationship of decolorization of dyes by ligninolytic activities produced by I. lacteus: MIP is more versatile in the decolorization of textile dyes and MnP activity is the only ligninolytic activity that is associated with the decolorizatio n of triphenyl methane dyes. RO16 is an azo dye with an intermediate compound vinyl sulphone azotized to 38 naphthalene (Fig. 3.3). RO13 is derived from an intermediate compound sulpho taubias acid azotized to naphthalene moiety and an additional intermediate, cyanuric chloride. Therefore, RO13 contains two naphthalene moieties (Fig. 3.3). Diazotization of vinyl sulphone group leads to the formation of RB5 (Fig 3.3). RBBR is an anthraquinone dye with a vinyl sulphone group (Fig 3.3). Presence of a halogenate d compound, cyanuric chloride, in the structure of RO13 makes it a suitable substrate for MIP and not for MnP. Whereas, MnP decolorizes RO16 that has naphthalene ring with hydroxyl group, presenting the dye as a simpler phenolic moiety. Reports on decolorization of sulfonphthalein dyes by ligninolytic enzymes of P. ostreatus has shown the preference of halogenated dye as a substrate for MIP and the non halogenated, simpler dyes as a preferential substrate for MnP (23, 25). RB5 has an additional vinyl sulphone group and is decolorized only by the MIP activity. The presence of additional vinyl sulphone group does not allow MnP or LIP to decolorize it. Decolorization of an anthraquinonic dye, RBBR, by I. lacteus suggests MIP as an important tool in degradation of xenobionts having anthraquinonic intermediates. MnP was the only ligninolytic activity associated to the decolorization of triphenyl methane dyes. Among the three triphenyl methane dyes, BG, FG and MB, BG resisted decolorization. The presence of two N, N-diethyl amine group in the structure of BG prevents the ligninolytic activities to use it as a substrate (Fig. 3.4). MB was decolorized better than the FG. Presence of three 4-amino benzene sulfonate group in MB, makes these dyes better substrates for Mn P (Fig. 3.4). FG has two 3-[2-(ethylamino) ethyl] benzene sulfonate group with one hydroxyl group and one sulfonic acid group attached to it. The order of preference of triphenyl methane dyes as substrate for MnP mediated decolorization is MB > FG. Since the effluents usually do not contain single dye but a concoction of dyes, therefore, a single enzymatic activity cannot bring about the degradation of effluents and thus there is a need to develop a blend of 39 enzymatic system that can act on wide varieties of dyes present in the effluent. NaO3SOCH2 N N S O Remazol Orange 3R C.I. Name: Reactive orange 16 C.I. No.: 17757 HO O CH3 C SO3Na SO3 Na NH OH N N N NaO 3S NaO3S NH N N CH3 N Cl Remazol Orange H2R C.I. Name: Reactive orange 13 C.I. No.: 18270 OH NaO3SOCH 2OS N N NH2 N N NaO 3S SO3Na Remazol Black B C.I. Name: Reactive black 5 C.I. No.: 20505 Remazol Brilliant Blue R C.I. Name: Reactive Blue 19 FW : 624.52 Figure 3.3 Structure of Textile dyes 40 SO 2CH 2CH 2OSO2Na CH3 CH3 N N CH3 Brilliant Green C.I. No: 42040 F.W.: 482.65 CH3 OH SO 3Na CH3 CH3 N N Fast Green C.I. No: 42053 F.W.: 808.86 SO3Na SO3 Na SO 3 Na HN HN SO3 Na NH Methyl Blue Acid Blue 93 F.W.: 799.8 SO 3 Na Figure 3.4 Structure of Triphenyl methane dyes 41 CHAPTER 4 ENZYMATIC DECOLORIZATION OF SULFONPHTHALEIN DYES 4.1 Introduction C chemical olor removal in particular has recently become of major scientific interest, as indicated by the multitude of related research reports (22, 122, 140, 143). Conventional waster water treatment is not efficient to remove recalcitrant dye stuffs from effluents. Physical and methods (adsorption, chemical transformation, incineration, photocatalysis and ozonation etc.) for the removal of dyes are not suitable due to high cost, low efficiency, and in-applicability to a wide variety of dyes (90, 111, 112, 144). Biodecolorization of paper pulp, paper waste-waters and synthetic dyes as measured by the decrease in color absorption was reported as early as 1980 (113). Lignin degrading fungi mineralize a number of important recalcitrant and xenobiotic compounds under ligninolytic conditions (16, 18, 21, 145, 146). WRF degrade xenobionts with varied capacity and thus researchers have stressed the need to perform a large scale screening of the ligninolytic enzyme systems of white rot fungi (68, 147, 148). Several screening methods involving dyes have been reported (26, 76, 149). Advantage of using WRF is that they do not require preconditioning to particular pollutants and its free radical based enzyme catalysis can degrade variety of xenobionts present in a very low concentration to non-detectable level and even complete elimination (150). The ability of these fungi to degrade lignin and structurally diverse pollutants results from the relatively non inducible and nonspecific nature of their extracellular enzymes (37, 151, 152). We tested a number of white rot fungi for their ligninolytic activity by using SP dyes as indicator substrate. In our report, we present the results of our 42 investigations of SP-decolorizing activity produced by P. ostreatus during solid state fermentation of wheat straw, a natural lignocellulosic substrate. 4.2 Materials and methods 4.2.1. Organism: Pleurotus ostreatus strain 3004 (ATCC 3545) originated from Culture Collection of Basidiomycetes (CCBAS), Prague was used (Kindly gifted by Šašek, V.). The strains were maintained on malt extract agar plates at 5 ºC. 4.2.2. Production of ligninolytic enzymes by P. ostreatus: Solid state fermentation of wheat straw by P. ostreatus was performed as described previously [12]. A set of 7 flasks was harvested on 5th day onwards. To the culture flasks 100 ml of phosphate buffer (0.1 M, pH 6.5) containing 0.1 M NaCl was added, contents were gently beaten and incubated on the rotary shaker for 1 h. Liquor obtained was then filtered and spun (6000 rpm, 15 min, 5oC) before being subjected to ammonium sulphate precipitation (80% saturation). Precipitates separated upon centrifugation (6000 rpm, 15 min, 5oC) were dissolved in the minimum amount of phosphate buffer (0.1 M, pH 6.5). Enzyme extract thus obtained was stored in small aliquots at -5ºC for further biochemical analysis. 4.2.3. Enzyme assays: Manganese peroxidase (MnP) and manganeseindependent peroxidase (MIP) activities were analyzed by oxidation of guaiacol to colored product using spectrophotometer. Reaction mixture (2 ml) for MnP activity contained 100 µmol succinate lactate buffer (pH 4.0), 0.2 µmol MnSO4, 0.9 µmol guaiacol, 0.7 µmol H2O2 and the enzyme extract. Reaction mixtures for MIP activity were similar to that of MnP but sodium tatarate buffer (pH 3.5) was used and MnSO4 was not added. One unit enzyme activity is defined as the amount of activity that produces a change of one A470 unit per min upon oxidation of guaiacol. 43 Sulfonphthalein (SP) dyes decolorizing activities of MnP and MIP were assayed using respective reaction mixtures as described above, wherein guaiacol was replaced by SP dyes investigated. MnII was varied in the range of 10-400 nmol in the reaction mixture. The reaction was terminated after 5 min with 1.25 µmol NaOH. Sodium tartarate buffer pH 3.5 and succinate lactate buffer pH 4.0 was used for the enzyme assay. Absorbance was monitored at the ?max of respective dyes. Dye decolorizing activity was calculated using molar absorbance coefficient of the respective dye (Table 4.1). One unit enzyme activity is defined as the amount of activity that decolorizes 1 nmol dye per min in the reaction mixture. 4.3 Results and discussion 4.3.1. Production of ligninolytic activities: Extracellular enzyme extracts prepared from Pleurotus ostreatus infested wheat straw possessed manganese peroxidase (MnP) and manganese-independent peroxidase (MIP) activities (Fig 4.1). MnP and MIP activities were produced throughout the experimental run since they were detectable even on day 1. These activities were maximal on day 7 and thereafter decreased gradually. Enzyme activity (U/L) 9 6 3 0 3 6 9 12 Days Figure 4.1 Manganese peroxidase activity at pH 3.5 (? ) and 4.0 (¦ ) and manganese-independent peroxidase activity at pH 3.5 (?) and 4.0 (?) of Pleurotus ostreatus growing on wheat straw 44 Manganese peroxidase (MnP; EC 1.11.1.13) was discovered in Phanerochaete chrysosporium and was later shown to be produced by other white rot fungi including P. ostreatus. The decolorization of dyes involving ligninolytic enzyme system of white rot fungi was first reported in 1983 and was used to measure ligninolytic activity of Ph. chrysosporium [76]. Along with the production of the ligninolytic activities, P. ostreatus also produces different dye decolorizing activities [26, 122]. We report the sulfonphthalein (SP) dyes decolorizing activities associated with MnP and MIP produced by P. ostreatus. Mechanisms involved in MnP-catalyzed reactions have been elucidated. It oxidizes MnII to MnIII, which is stabilized by organic acid chelators and acts in turn as a low molecular mass, diffusible, redox mediator that attacks organic molecules nonspecifically via hydrogen and one electron abstraction [32, 153]. 4.3.2. Decolorization of sulfonphthalein dyes: The SP dyes (Fig 4.2) were decolorized at pH 4.0 in the absence of MnII and the decolorizing activity increased concomitantly with the increase in the concentration of MnII in the reaction mixture. Enhancement in the sulfonphthalein decolorizing activity (SPDCA) by MnII is attributed to the MnP activities. However, MnII was observed to inhibit 5th and 7th day bromophenol red (BPR) decolorizing activity (Fig. 4.3 and 4.4), 7th day bromocresol green (BCG) decolorizing activity (Fig. 4.4), and 5th, 7th and 11th day bromophenol blue (BPB) decolorizing activity (Fig 4.3, 4.4 and 4.6). MnP of Bjerkandera adusta and Pleurotus eryngii decolorized azo and heterocyclic dyes in a MnII-independent manner [90]. Two interesting observations in this context were made pertaining enzymatic decolorization of BPB, BCG and BPR. First, decolorization occurred in the absence of MnII. Second, the inhibition of the decolorization reaction was proportionate to the concentration of MnII. Decolorization of BPB, BCG and BPR in the absence of MnII and inhibition by MnII led us to investigate the role of MIP in the SP-DCA. 45 Inhibitory influence of MnII on the decolorization of SP dyes was stronger and prominent at pH 3.5 than at 4 and was a function of MnII conc (Fig 4.3-4.6). Halogenated SP dyes (BCG, BCP and BPB) are poorer substrates of MnP and the extent of inhibition in the presence of MnII increased with the degree of halogenation of these dyes (Fig 4.3-3.6). O S R2' O R1' O R1 R2 OH OH R3' Sulfonphthalein R3 R1=R 1' R2=R 2' R3=R 3' Bromocresol green CH3 Br Br Bromocresol purple H CH3 Br Bromophenol blue H Br Br Bromophenol red H Br H CH3 H H o-Cresol red H H CH3 Phenol red H H H dyes m-Cresol purple Figure 4.2 General structure of sulfonphthalein dyes 46 MIP decolorized BPB better than BCP and BCG. MIP -catalyzed decolorization of phenol red (PR), ortho-cresol red (o-CR) and meta-cresol purple (m-CP) was less than the halogenated SP chromophores at pH 3.5 (Fig 4.3-4.6). The increase in the degree of halogenation of SP dyes makes them better Sulfonphthalein dye decolorizing activity (U/L) substrates for MIP. Figure 4.3 Influence of MnII on the sulfonphthalein decolorizing activity at pH 3.5 and 4.0 extracted from 5-day old wheat straw infested by Pleurotus ostreatus MnP-catalyzed decolori zation of o-CR is better than m-CP. This indicates that methyl group in ortho position (o-CR) is favorable than in meta position (mCP) for the decolorization reaction (Fig 3.2). The apparent Km values (Table 4.1) 47 of o-CR (26 mM) and m-CP (40 mM) provided the evidence of influence of methyl group on the decolorization of the two dyes by MnP. Thus position of auxochrome on SP chromophore is important for the decolorization. MnP-catalyzed decolorizing activity of BCP was less than o-CR. Bromine group on ortho position (BCP) was less effective than methyl group in ortho position (o-CR) on the chromophore (Fig 4.2) for the decolorization by MnP. This Sulfonphthalein dye decolorizing activity (U/L) is evident from the higher K m for BCP (36 mM) and lower K m for o-CR (26 mM). Figure 4.4 Influence of MnII on the sulfonphthalein decolorizing activity a t pH 3.5 and 4.0 extracted from 7-day old wheat straw infested by Pleurotus ostreatus 48 An additional bromine group on SP chromophore further impeded the decolorizing ability of MnP. BPB and BCG (having two bromine) were decolorized less than BCP (having one bromine) as evident from the kinetic constants (Table 4.1). Kinetic constants (V max and Km) indicated that PR which has low values of Km is catalytically more favorable for decolorization by MnP Sulfonphthalein dye decolorizing activity (U/L) (Table 4.1). PR served as a poor substrate for MIP. Figure 4.5 Influence of MnII on the sulfonphthalein decolorizing activity at pH 3.5 and 4.0 extracted from 9-day old wheat straw infested by Pleurotus ostreatus The order of preference of SP dyes as substrate is reverse in case of MnPand MIP-catalyzed decolorization. Free phenolic group present on the 49 chromophore of SP dyes (Fig 4.2) make them susceptible to attack by MnP whereas presence of halogen (bromine) group (Fig 4.2) on SP chromophore formulate them as a better substrate for MIP. Thus position, type and numbers of the auxochromes on SP chromophores (Table 3.1) affect the enzymatic decolorization of SP dyes. MIP appears to be a better candidate than MnP for the Sulfonphthalein dye decolorizing activity (U/L) degradation of halogenated aromatic compounds. Figure3.6 Influence of MnII on the sulfonphthalein decolorizing activity at pH 3.5 and 4.0 extracted from 11-day old wheat straw infested by Pleurotus ostreatus 50 Table 4.1 Kinetic constants for sulfonphthalein dyes of manganese peroxidase produced by Pleurotus ostreatus during solid-state fermentation of wheat straw Sulfonphthalein λmax ε Km Vmax dyes (nm) (M-1 cm-1) (mM) (U/ml) Bromocresol green (BCG) 621 39569 117 0.125 Bromocresol purple (BCP) 593 60865 36 1 Bromophenol blue (BPB) 583 52663 78 0.066 Bromophenol red (BPR) 564 10735 69 0.065 m-Cresol purple (m-CP) 582 32406 40 0.133 o-Cresol red (o-CR) 577 41931 26 0.286 Phenol red (PR) 564 30737 16 0.057 PR-DCA (U/L) 100 75 50 25 0 0 5 10 15 NaN3 (µM) Figure 4.7 Influence of sodium azide on Phenol red decolorizing activity (PRDCA) by manganese-independent peroxidase at pH 3.5 (¦ ) & 4.0 (? ) and manganese peroxidase at pH 3.5 (?) & 4.0 (?) White rot fungi characteristically produce several isozymes of ligninolytic enzymes [22]. Variations in the enzymatic decolorization of SP dyes by these 51 activities produced on different days reflect differences in the composition of isozymes of MnP and MIP activities. Difference (kinetics constants and also substrate specificity) in the decolorization of SP dyes by enzyme preparations of various days substantiates the observation made above (Fig 4.3-4.6). NaN3, an oxidase inhibitor, was employed for the assessment of the nature of decolorizing reactions of SP dyes. NaN3 inhibited the phenol red decolorizing activity (PR-DCA) by MnP at pH 4.0. PR proved to be a better substrate for MnP (Fig 3.7). This explained the oxidative nature of SP-decolorization reaction catalyzed by MnP and MIP. Chapter Published: Shrivastava, R., Christian, V., and Vyas, B. R. M. (2005) Enzymatic decolorization of sulfonphthalein dyes. Enz. Microb. Technol. 36: 333337 52 CHAPTER 5 HORSERADISH PEROXIDASE: DECOLORIZATION OF TEXTILE AND TRIPHENYL METHANE DYES 5.1 Introduction T he word "horse" (as applied in "horseradish") is believed to denote large size and coarseness. "Radish" comes from the Latin radix meaning root. Horseradish peroxidase (E.C.1.11.1.7) (HRP) is a heme protein with molecular weight of 40,000 Daltons and optimal pH 7.0 (154). Seven isozymes of HRP have been reported (155-157). All contain photohemin IX as prosthetic group. Neutral and amino sugars account for approximately 18% of the enzyme. Horseradish peroxidase catalyzes oxidation of ascorbate, ferrocyanide, cytochrome C and the leuco form of many dyes; the reaction mechanism observed is as follow HRP + H2O2 ? Compound I Compound I + AH2 (reduced substrate) ? Compound II + AH? Compound II + AH2 ? HRP + AH 2AH ? Oxidized Product Bioremediation is a pollution control technology that uses biological systems to catalyze the degradation or transformation of various toxic chemicals to less harmful forms. The general approaches to bioremediation are to enhance natural biodegradation by native organisms (intrinsic bioremediation), to carry out environmental modification by applying nutrients or aeration (bio-stimulation) or through addition of microorganisms (bio-augmentation). Unlike conventional technologies, bioremediation can be carried out on-site. Bioremediation is limited in the number of toxic materials it can handle, but where applicable, it is cost- 53 effective (158). Horseradish peroxidase is known to degrade recalcitrant organic compounds such as phenol, substituted phenols and various synthetic dyes (159). Present study focuses on the ability of HRP to degrade dyes, model of xenobionts, of varied classes (textile and triphenyl methane). 5.2 Materials and Methods 5.2.1. Enzyme: Horseradish peroxidase (HRP) was obtained from SRL Pvt. Ltd. The purified enzyme was preserved at -20oC. 5.2.2. Textile Dyes: Reactive black 5, Remazol black HR, Reactive orange 13, Reactive orange 16, Remazol red H8B, Remazol magenta HB, Remazol turquoise blue G, Remazol lemon yellow F4, Remazol golden yellow R, Reactive brown 18 and Remazol brilliant blue R were obtained from the dye manufacturing and textile processing industries. 5.2.3. Triphenyl methane Dyes: Brilliant green, Methyl blue and Fast green were procured from Hi-Media (India). 5.2.4. Enzyme assays: Reaction mixture (2 ml) for HRP contained 100 µmol sodium tartarate buffer (pH 2.5, 3.0 and 3.5), succinate lactate buffer (pH 4.0 and 4.5) and sodium citrate buffer (pH 5.0 and 5.5), 150 ppm textile dye or 75 ppm triphenyl methane dyes, 0.4 µmol H2 O2 and the purified enzyme. One unit enzyme activity is defined as the amount of activity that consumes 1 µmol of the triphenyl methane dye or produces a change of one absorbance unit per min upon oxidation of textile dye at its λmax. 5.3 Results and Discussion Horseradish peroxidase (HRP) derived from Ipomea palmata and Saccharum spontaneum has been shown to degrade wide variety of textile dyes and anthraquinonic dye, RBBR (159, 160). We investigated the decolorization of 54 eleven textile dyes and three triphenyl methane dyes by commercially available purified HRP. 5.3.1. Decolorization of triphenyl methane dyes: Triphenyl methane dyes, methyl blue (MB) and fast green (FG), were decolorized by Horseradish peroxidase (HRP). Brilliant green (BG) resisted decolorization by HRP (Fig 5.1). Methyl blue was the best decolorized triphenyl methane dye by HRP. Decolorization of MB occurred above pH 4 and decolorization remained constant above pH 5. Decolorization of FG also occurred above pH 4 and it increased till pH 5.5. Decolorization of FG suggests the decolorization process needs to be studied over a broader pH range. 30 BG 20 10 % Decolorization 0 30 MB 20 10 0 15 FG 10 5 0 2 3 pH 4 5 6 Figure 5.1 Decolorization of triphenyl methane dyes Brilliant green (BG), Methyl blue and (MB), and Fast green (FG) by horseradish peroxidase. 55 5.3.2. Decolorization of textile dyes: Horseradish peroxidase decolorized five of the textile dyes investigated, namely, reactive black 5 (RB5), reactive orange 16 (RO16), remazol brilliant blue R (RBBR), remazol turquoise blue G (RTBG), and remazol red H8B (RRH8B). Remazol black HR (BHR), reactive orange 13 (RO13), remazol magenta HB (RMHB), remazol lemon yellow F4 (LYF4), remazol golden yellow R (GYR), reactive brown 21 (RB21), were not decolorized by the HRP (Fig. 5.2). Decolorization of RB5 occurred above pH 3.5 which increased beyond pH 4.5 and remained constant till pH 5.5. Similar results were obtained with RBBR (Fig 5.2). Remazol red H8B (RRH8B) was decolorized over the pH range of 3.5 to 5.5. The increase in the decolorization of RRH8B was the function of pH, with the rise in the pH the decolorization also increased substantially. Decolorization of the dyes, RO16 and RTBG, occurred above pH 4.5 and 5, respectively. 5.3.3. Influence of pH: Decolorization of textile dyes and triphenyl methane dyes by HRP occurred over a broad range of pH 3.5-5.5. But the significant decolorization, in most of the cases, was at the optimal pH of HRP. Textile dyes, RO16 and RTBG, and triphenyl methane dye, FG, were strict in pH selection for decolorization by HRP. Their decolorization occurred at particular pH (4 or 4.5). This implies that in scheming bioremediation, recognizing optimal pH for certain class of dyes is must and under defined pH only the system can be made efficient. One of the two black dyes, RB5 was decolorized but not the other, BHR. Similarly, of orange dyes, RO16 and RO13, only RO16 was decolorized. Similar observation was also made in the case of green triphenyl methane dyes, BG and FG, where, FG was decolorized but BG resisted decolorization. The investigations done by Pasti-Grigsby et. al. have attributed the reason to be the 56 30 30 RB5 20 20 10 10 0 0 30 30 RO16 20 20 10 10 0 0 80 BHR RO13 30 RBBR 60 RTBG 20 40 10 20 0 0 30 30 RRH8B 20 20 10 10 0 0 30 RMHB 30 LYF4 20 20 10 10 0 0 GYR 2 30 3 pH 4 5 6 BrH4R 20 10 0 2 3 pH 4 5 6 Figure 5.1 Decolorization of textile dyes by horseradish peroxidase (?):Reactive black 5 (RB5); Remazol black HR (BHR); Reactive orange16 (RO16); Reactive orange 13 (RO13); Remazol brilliant blue R (RBBR); Remazol turquoise blue G (RTBG); Remazol red H8B (RRH8B); Remazol magenta HB (RMHB); Remazol lemon yellow F4 (LYF4); Remazol golden yellow R (GYR); Reactive brown 18 (RB18) 57 differences in the electron distribution and charge density along with the stericdistribution in different dyes (126). 5.3.4. Structure function relationship of decolorization of dyes by Horseradish peroxidase: Among the three triphenyl methane dyes, FG and MB was decolorized and BG resisted decolorization by HRP. MB has three 4-amino benzene sulfonate group and presence of this group makes MB decolorize better compared to FG by HRP (Fig. 3.4, Chapter 3). Presence of two 3-[2-(ethylamino) ethyl] benzene sulfonate group with one hydroxyl group and one sulfonic acid group in FG makes this dye a substrate for HRP. The presence of two N,Ndiethyl amine group in the structure of BG prevents the HRP to use it as a substrate (Fig. 3.4, Chapter 3). The selection of triphenyl methane dyes as a substrate for decolorization by HRP is similar to that of manganese peroxidase of Irpex lacteus (Chapter 3). The order of preference of triphenyl methane dyes as substrate for HRP mediated decolorization is MB > FG. Among eleven textile dyes investigated for decolorization, HRP was able to decolorize five of the dyes namely, reactive black 5 (RB5), reactive orange 16 (RO16), remazol brilliant blue R (RBBR), remazol turquoise blue G (RTBG), and remazol red H8B (RRH8B). Between two orange dyes, RO16 and RO13, RO16 was decolorized whereas, RO13 resisted decolorization by HRP. RO16 has an intermediate compound vinyl sulphone azotized to naphthalene and RO13 has an intermediate compound sulpho taubias acid azotized to naphthalene moiety and an additional intermediate, cyanuric chloride (Fig. 3.3, Chapter 3). Azotization of vinyl sulphone intermediate to naphthalene is favourable for decolorization by HRP than azotization of sulpho taubias acid. Diazotization of vinyl sulphone group leads to the formation of RB5 (Fig. 3.3, Chapter 3). Therefore, presence of vinyl sulphone group as an intermediate in dye (like, RB5 and RO16) and absence of any halogenated intermediate (for example cyanuric chloride) favours the decolorization of dye by HRP. 58 RBBR is an anthraquinone dye with a vinyl sulphone group (Fig. 3.3, Chapter 3). Decolorization of an anthraquinonic dye, RBBR, by HRP is suggestive of HRP as an important tool in degradation of xenobionts having anthraquinonic intermediates. The selection of dye as a substrate for decolorization by HRP is similar to that of manganese peroxidase of Irpex lacteus. HRP preferred the dyes with simple structure and dyes that do not have halogenated intermediates. The dye having the simple phenolic moiety is decolorized better by HRP. Thus, HRP and its reaction mechanism in degradation of dyes need due attention as a candidate and in defining the strategies for the development of promising industrial wastewater treatment system. 59 CHAPTER 6 LIGNINOLYTIC ENZYMES OF IRPEX LACTEUS: DECOLORIZATION OF EFFLUENTS 6.1 Introduction W hite-rot basidiomycetes (WRB) produce various isoforms of extracellular oxidases namely laccase, manganese peroxidase (MnP) and lignin peroxidase (LIP ) that are involved in the degradation of lignin in the natural lignocellulosic substrates (37). This ligninolytic system of WRB is directly involved also in the degradation of various xenobiotics compounds and dyes (145, 146, 151, 152). The textile industry, by far the most avid user of synthetic dyes, is in need of eco-efficient solutions for its colored effluents. Chemical products used in textile processing affect the environment by their effluents that can be voluminous, colored and varied (74, 109). Thoug h, the physical and chemical methods offer some solutions to the problems, it is not affordable by the unit operators. Biological degradation is recognized as the most effective and ecofriendly method for degrading the dyes present in the wastes. Research over a period of two decades has provided insight into the nonspecific enzyme system of white rot fungi, which oxidizes the recalcitrant dyes (162). The decolorization and detoxification potential of WRB can be harnessed; thanks to the emerging knowledge of the physiology and enzymology of these organisms. The knowledge gathered needs to be transformed into reliable and robust waste treatment processes. This chapter evaluates prospective use of ligninolytic enzymes of WRB, Irpex lacteus, for the treatment of industrial effluents, particularly dye containing effluents. 60 6.2 Materials and Methods 6.2.1. Microorganism: Irpex lacteus Fr. 238 617/93 obtained from Culture Collection of Basidiomycetes (CCBAS), Prague (Kind gift from V. Šašek). The strain is maintained on malt extract agar plates at 4 ºC. 6.2.2. Effluents: Effluents, E1 and E2, were obtained from dyeing and printing industries located in Jetpur, Gujarat. 6.2.3. Production of ligninolytic enzymes by I. lacteus: Solid state fermentation of wheat straw by I. lacteus was performed as described previously [136]. To the culture flasks, harvested on 18th day, 100 ml of phosphate buffer (0.1 M, pH 6.5) containing 0.1 M NaCl was added, contents were gently beaten and incubated on rotary shaker for 1 h. Liquor obtained was then filtered and spun (6000 rpm, 15 min, 5oC) before being subjected to ammonium sulfate precipitation (80% saturation). Precipitates separated upon centrifugation (6000 rpm, 15 min, 5oC) were dissolved in the minimum amount of phosphate buffer (0.1 M, pH 6.5). Enzyme extract thus obtained was desalted using PD10 column (Pharmacia) and stored in small aliquots at -5ºC for further biochemical analysis. 6.2.4. Enzyme assays: Manganese peroxidase (MnP) and manganeseindependent peroxidase (MIP) activities were determined as described by Vyas et al. (136). Lignin peroxidase activity was estimated according to Tien and Kirk (34). One unit of enzyme activity is defined as the amount of activity that will produce one µmol of the product per min upon oxidation of the substrate in the reaction mixture under the assay conditions. 6.2.5. Effluent decolorizing enzyme assays: Effluent decolorizing activity of MnP, MIP and LIP was assayed using respective reaction mixtures. Reaction mixture (2 ml) for MnP activity contained 100 µmol buffer, 0.2 µmol MnSO4 , 150 ppm effluents, 0.4 µmol H2O2 and the enzyme extract. Sodium tartarate buffer for pH 2.5, 3.0 and 3.5, succinate lactate buffer for pH 4.0 and 4.5, and sodium 61 citrate buffer for pH 5.0 and 5.5 were used. Reaction mixture for MIP activity was similar to that of MnP but MnSO4 was not added. Reaction mixture for LIP activity was similar to that of MnP but veratryl alcohol (4 µmol) was added instead of MnSO4 . One unit enzyme activity is defined as the amount of activity that produces a change of one absorbance unit per min upon oxidation of textile dye at its λ max. 6.3 Results and Discussion The ligninolytic system of white-rot basidiomycetes (WRB) is directly involved in the degradation of many xenobionts. The decolorization of dyes as well as its use to measure ligninolytic activities was first reported for Phanerochaete chrysosporium [76]. Thereafter, Pleurotus ostreatus has also been shown to produce different dye decolorizing activities [25, 26, 122]. We report the effluents decolorizing activities associated with manganese peroxidase (MnP), manganese-independent peroxidase (MIP) and lignin peroxidase (LIP) produced by Irpex lacteus. 6.3.1. Influence of pH on ligninolytic enzyme activities: Extracellular enzyme extracts prepared from Irpex lacteus-infested wheat straw possessed MnP MIP and LIP activities (Fig 6.1). The optimal pH of MnP was observed to be 4 and that of LIP was 3. MIP activity was maximal at pH 3.5 and thereafter decreased gradually. Enzyme activity (U/l) 300 200 100 0 2 2.5 3 pH 3.5 4 4.5 Figure 6.1 MnP activity (-?-), MIP activity (-?-) and LIP activity (-?-) at varying pH of Irpex lacteus growing on wheat straw. 62 6.3.2. Influence of H2O2 on ligninolytic enzyme activities: All the ligninolytic activities showed positive influence to the increasing concentration of hydrogen peroxide (H2O2). MnP and MIP activities were maximum at the final concentration of H2 O2 (100 µM) examined (Fig. 6.2). LIP activity was also maximal at 500 µM of H2O2 (Fig. 6.2). Mechanisms involved in MnP-catalyzed and LIP -catalyzed reactions have been elucidated (32, 105). H2O2 , the primary substrate of MnP, generates oxidized species of MnP (2 electron deficient Compound I) that oxidizes MnII to MnIII which is stabilized by organic acid chelators. Similarly LIP Compound I generated in the presence of H2 O2 oxidizes veratryl alcohol to veratryl alcohol cation radical. MnIII and veratryl alcohol cation radical in turn act as low molecular mass, diffusible oxidants attacking organic molecules nonspecifically via hydrogen and one electron abstraction [32, 105, 153]. MnP (U/ml) 1 0.75 0.5 0.25 0 0 25 50 H2 O2 (µM) 75 100 MIP (U/ml) 0.5 0.25 0 0 25 50 H2O2 (µM) 63 75 100 LIP (U/ml) 0.15 0.1 0.05 0 0 100 200 300 400 500 H2O2 (µM) Figure 6.2: Influence of H2 O2 on ligninolytic enzyme activities produced by Irpex lacteus growing on wheat straw 6.3.3. Decolorization of Effluent E1 by ligninolytic enzyme activities: Ligninolytic activities investigated decolorized the effluent E1 completely. MnP was found to be the best ligninolytic activity associated with decolorization of effluent E1 (Fig. 6.3). MIP was better than LIP in the decolorization of effluent E1. During the enzymatic decolorization absorbance in the UV-region decreased drastically and ultimately resulted in the complete loss of the peak in the UVregion. Decolorization pattern did not change in the pH range 3-5.5. The pH of the reaction mixture did not exert any influence on decolorization of E1 by the ligninolytic activities. The physico-chemical properties of E1 are mentioned in Table 6.1. 6.3.4. Decolorization of effluent E2 by ligninolytic enzyme activities: Ligninolytic activities investigated also decolorized the effluent E2 completely. MnP and MIP were found to be the better ligninolytic activities associated with the decolorization of effluent E2 (Fig. 6.4). LIP also contributed to the decolorization of effluent E2. Similar to the decolorization of E1, during the decolorization of E2 simultaneous loss of the peak in UV-region was observed. Decolorization of E2 by the ligninolytic activities, MnP and MIP , was not influenced by the pH of the reaction mixture. LIP -associated decolorization of E2 64 showed loss in the efficiency with the increasing pH. The physico-chemical properties of E2 are mentioned in Table 6.1. 4 pH 3 2 4 pH 4.5 0 2 4 Absorbance Absorbance pH 3.5 2 0 4 pH 5 0 2 4 pH 4 0 150 350 550 750 wavelength (nm) 2 0 150 350 550 750 wavelength (nm) Figure 6.3 UV-visible spectra of effluent E1 before (??? ) and after incubation with MnP (………), MIP (- - - -) and LIP (-·-·-·-) 65 4 pH 3 2 4 pH 4.5 0 2 4 Absorbance Absorbance pH 3.5 2 0 4 pH 5 0 2 4 pH 4 0 150 2 350 550 750 wavelength (nm) 0 150 350 550 750 wavelength (nm) Figure 6.4 UV-visible spectra of effluent E2 before (??? ) and after incubation with MnP (………), MIP (- - - -) and LIP (-·-·-·-) 66 Table 7.1 Physico-chemical characterization of effluents (E1 and E2) from dyeing and printing industries Physico-chemical E-1 E-2 pH 6.45 6.8 Conductivity 5.79 5.24 Turbidity (NTU)a 0.4 1.3 solids 440 360 Copper (ppm) 0.022 0.081 Nitrite (ppm) 26 38 Zinc (ppm) 2.9 3.9 Chloride (ppm) 38 86 Sulfate (ppm) 666.4 780.9 parameter Total dissolved Ligninolytic activities investigated decolorized the effluents, E1 and E2 completely. The two major differences observed in the decolorization of E1 and E2 were, firstly, the ligninolytic activities, MnP was the only activity that decolorized E1 better than any other ligninolytic activities investigated, whereas, in the case of E2, MnP and MIP were the activities that decolorized E2 better. Secondly, the ligninolytic activities associated to decolorization of E1 showed no influence of pH and decolorized the effluent over the range of pH of investigated, whereas, efficiency of LIP to decolorize E2 decreased with the rise in pH. The differences in the decolorization of E1 and E2 can be attributed to the differences in their physico-chemical properties. Though the pH has high impact on ligninolytic activities, as in case of MnP where the pH at which the enzyme is stable is 7 and the pH at which it is highly active is 4 or 4.5 but the pH in MnP and MIP associated decolorization of effluents, E1 and E2, had no influence and the effluents were decolorized with same efficiency over the range of pH investigated. 67 CHAPTER 7 D ECOLORIZATION OF DYES AND EFFLUENTS BY FENTON AND FENTON-LIKE R EAGENTS 7.1 Introduction M any metals have special oxygen transfer properties which improve the utility of hydrogen peroxide. By far, the most common of these is iron which, when us ed in the prescribed manner, results in the generation of highly reactive hydroxyl radicals (•OH). The Fenton's reaction has been known since 1894 by its inventor H.J.H. Fenton (61) and is currently one of the most powerful oxidizing reactions available. The reaction involves hydrogen peroxide and a ferrous iron catalyst. The peroxide is broken down into a hydroxide ion and a hydroxyl free radical. The hydroxyl free radical is the primary oxidizing species and can be used to oxidize and break apart organic molecules. [1] Fe II+ + H2O2? OH· + Fe III+ OH– The common pathways for the white-rot fungal production of Fenton’s reagents are mentioned below (107). [2] 2Fe III+ + –O2C·CO2 – ? 2FeII+ + 2CO2 [3] FeII+ + O 2 ? Fe III+ + O2·– [4] Fe II++ O 2· – + 2H + ? Fe III+ + H2O 2 – [5] O 2· + H2O2 ? OH· + OH – + O2 Toxic and refractory organic pollutants in industrial wastewater can be degraded by advanced oxidation processes alone or in combination with physico-chemical and biological processes (163). Of these oxidation methods, Fenton's reagent (FeII/H2O 2) and Fenton-like reagents (CuII/H2O2), are effective oxidants of large variety of organic pollutants. Fenton reagents are also produced from diverse routes by lignin-degrading fungi (107). The mechanism of decomposition of H2O2 and of oxidation of organic solutes by Fenton's and Fenton-like reactions has been the subject of numerous studies 68 (163, 164). A recent study however, reported decolorization of synthetic dyes by Fenton system (165). In our report, we present the results of our investigations of decolorization of textile, triphenyl methane dye and effluents from dyeing and printing industries by Fenton Reagent (FeII/H2O 2) and Fenton-like reagents (CuII/H2O2). 7.2 Materials and Methods 7.2.1 Chemicals: FeII as FeSO4, CuII as CuSO4 and hydrogen peroxide were added as aqueous solutions to the reaction mixture. 7.2.2 Textile Dyes : Reactive black 5, Remazol black HR , Reactive orange 13, Reactive orange 16, Remazol red H8B, Remazol magenta HB, Remazol turquoise blue G, Remazol lemon yellow F4, Remazol golden yellow R, Reactive brown 18 and Remazol brilliant blue R were obtained from the dye manufacturing and textile processing industries. 7.2.3. Triphenyl methane Dyes: Brilliant green, Methyl blue and Fast green were procured from Hi-Media (India). 7.2.4. Effluents : Effluents, E1 and E2, were obtained from various dyeing and printing industries. 7.2.5. Decolorization of dyes: Textile dyes (150 ppm), triphenyl methane dyes (75 ppm) and effluents (3/4 th of effluent diluted to 1/4th by distill water) were added to the reaction mixture as aqueous solutions. Reaction mixture (2 ml) for Fenton reagent contained 1 mM FeSO4 and 10 mM hydrogen peroxide. Reaction mixture for Fenton-like reagent was similar to that of Fenton reagent but contained 1 mM CuSO4. The dyes were incubated with Fenton and Fenton-like reagent and scanned in the range of 200-800 nm, before and after decolorization using Shimadzu UV-1601 spectrophotometer. The decolorization was initiated by the addition of hydrogen peroxide to the reac tion mixture. The incubation proceeded at 18 °C in the dark. 69 7.3 Results and Discussion Ligninolytic enzyme system of white-rot basidiomycetes (WRB) generates hydroxyl radical, a reactive oxygen species, which plays important role in wood decay. Hydroxyl radical is the strongest oxidant that occurs in aqueous system. It reacts with all organic molecules by abstracting hydrogen from aliphatic structures or by adding an electrophile to aromatic structures (166). Hydroxyl radical is found to be generated in all the three major types of wood decay caused by white -rot, brown-rot and soft-rot fungi (163, 167, 168). 7.3.1. Decolorization of textile dyes by Fenton reagent and Fenton-like reagent: Textile dyes were completely decolorized by Fenton reagent (Fig. 7.1). Decolorization initiated immediately with the addition of hydrogen peroxide. Fenton reagent-mediated decolorization of dyes led to the loss of absorbance maxima in visible region and decrease in the absorbance in UVregion. Textile dye, Remazol turquois e blue G (RTBG), was decolorized less as compared to the other dyes. Decolorization of dyes, remazol brilliant blue R (RBBR), reactive orange 13 (RO13), remazol red H8B (RRH8B), RTBG, and remazol black HR (BHR) resulted in the generation of new peak in the UV-region. Textile dyes were completely decolorized by Fenton-like reagent (Fig. 7.1). Dyes, RO13, Remazol lemon yellow F4 (LYF4), Reactive brown 18 (RB18) and BHR, resisted immediate decolorization by Fenton-like reagent. Decolorization of textile dyes by Fenton-like reagent led to the generation of new peaks in the visible as well as in the UV-region. The new peaks, generated as a part of decolorization reaction, shifted towards the lower wavelength. It is important to report that decolorization of the some of the dyes, reactive orange 16 (RO16), RRH8B, and LYF4, initiated with the increase in absorption, shifting the peak towards the higher wavelength. The reports on decolorization of dyes by white-rot fungi have shown the process of oligomerization before degradation (169). 70 4.5 RBB RB 5 Absorbance Absorbance 4.5 3.0 1.5 0.0 200 400 600 3.0 1.5 0.0 200 800 RBBR 400 RO13 RO3R 4.5 Absorbance Absorbance 4.5 3.0 1.5 0.0 200 400 600 600 3.0 1.5 0.0 200 800 RO16 ROH2R 400 λ (nm) 4.5 RRH8B 3.0 1.5 0.0 200 400 600 800 4.5 Absorbance Absorbance 800 1.5 400 λ (nm) RTBG 1.5 400 600 3.0 0.0 200 3.0 0.0 200 800 RMHB λ (nm) 4.5 600 λ (nm) Absorbance Absorbance 4.5 800 λ (nm) λ (nm) 600 3.0 1.5 0.0 200 800 λ (nm) LY LYF4 400 600 λ (nm) 71 800 4.5 GYF4 GYR Absorbance Absorbance 4.5 3.0 1.5 0.0 200 400 600 3.0 1.5 0.0 200 800 BRH4R RB18 400 4.5 Absorbance 600 800 λ (nm) λ (nm) BHR 3.0 1.5 0.0 200 400 600 800 λ (nm) Figure 7.1 UV-visible spectra of textile dyes before (? ? ? ) and after 20 min of incubation with Fenton reagent (- - - -) and Fenton-like reagent (………): Reactive black 5 (RB5); Remazol brilliant blue R (RBBR); Reactive orange 13 (RO13); Reactive orange 16 (RO16); Remazol red H8B (RRH8B); Remazol magenta HB (RMH B); Remazol turquoise blue G (RTBG); Remazol lemon yellow F4 (LYF4); Remazol golden yellow R (GYR); Reactive brown 18 (RB18); Remazol black HR (BHR). 7.3.2. Decolorization of triphenyl methane dyes by Fenton reagent and Fenton-like reagent: The three triphenyl methane dyes investigated were completely decolorized by the Fenton reagent. The decolorization of the dyes, brilliant green (BG), methyl blue (MB) and fast green (FG) was similar to the most of the textile dyes (Fig. 7.2). Decolorization proceeded with the loss of peaks in the visible as well as decrease in the absorbance in UV-region. Among the triphenyl methane dyes, BG was the only dye that was decolorized by the Fenton-like reagent. Decolorization of BG by Fenton-like reagent proceeded slowly (Fig. 7.2). The phenomena of oligomerization as 72 4.5 BG Absorbance Absorbance 4.5 3.0 1.5 0.0 200 400 600 MB 3.0 1.5 0.0 200 800 400 λ (nm) 4.5 Absorbance 600 800 λ (nm) FG 3.0 1.5 0.0 200 400 600 800 λ (nm) Figure 7.2 UV-visible spectra of triphenyl methane dyes before (??? ) and after 20 min of incubation with Fenton reagent (- - - -) and Fenton-like reagent (………): Brilliant green (BG); Methylene blue (MB); Fast green (FG). observed in the decolorization of some of the textile dyes, was also experiential in the decolorization of BG. Therefore, Fenton reagent is more viable option in degradation studies concerning triphenyl methane dyes. 7.3.3. Decolorization of effluents by Fenton reagent and Fenton-like reagent: Despite of difference in the physico-chemical properties (Table 7.1) of the effluents, E1 and E2, they were decolorized efficiently by both the systems, Fenton reagent and Fenton-like reagent (Fig. 7.3). Decolorization of the effluents followed by loss in absorbance in visible as well as UV-region. Fenton-like reagent was more efficient in decolorization of effluent E2. 73 4.5 E1 Absorbance Absorbance 4.5 3.0 1.5 0.0 200 400 600 3.0 1.5 0.0 200 800 E2 400 600 800 λ (nm) λ (nm) Figure 7.3 UV-visible spectra of effluents before (? ? ? ) and after 20 min of incubation with Fenton reagent (- - - -) and Fenton-like reagent (………): Effluent 1 (E1); Effluent 2 (E2). Decolorization of E1 by both the systems investigated showed resistance, but E2 was decolorized better when compared to the former. The better decolorization of E2 can be attributed to its physico-chemical properties (Table 7.1). The conductivity and total dissolved solids of E2 were lower when compare to E1. The pH of the E2 was higher, towards neutral, and this assisted in rapid decolorization of E2 by both the systems investigated. It has been reported that pH plays a very vital role in decolorization by redox coupling system like, Fenton reagent (108, 164). Decolorization of dyes and effluents by Fenton reagent and Fenton-like reagent initiates with the addition of hydrogen peroxide. Dyes and effluents, under investigation, were decolorized completely by Fenton reagent and Fenton-like reagent and showed hypsochromic shift as well as appearance of new peak towards lower wavelength (Fig. 1-3). Shift in the absorption maxima, in both the cases, during decolorization of dyes implicates the process to be occurring in steps where intermediates are generated. Similar results were obtained with decolorization by modified Fenton reagent using Cu/pyridine/hydrogen peroxide (108). Hypsochromic shift is suggestive of degradation of the molecule through a series of intermediates (26, 149). 74 Table 7.1 Physico-chemical characterization of effluents (E1 and E2) from dyeing and printing industries Physico-chemical parameter E-1 E-2 6.45 6.8 5.79 5.24 0.4 1.3 Total dissolved solids 440 360 Copper (ppm) 0.022 0.081 Nitrite (ppm) 26 38 Zinc (ppm) 2.9 3.9 Chloride (ppm) 38 86 Sulfate (ppm) 666.4 780.9 pH Conductivity Turbidity (NTU) a Fenton reagent is better than Fenton-like reagent for the decolorization of dyes; firstly, Fenton reagent decolorized the dyes rapidly compare to Fenton-like reagent, secondly, Fenton reagent is more powerful redox system as it was able to decrease the absorption even in the UV -region, thirdly, Fenton reagent is very efficient in programming the remediation of textile effluents as it was able to decolorize most of the dyes completely. Redox system and mechanism of Fenton reagent needs more attention to develop it as a potent tool against the water pollution abatement programs. 75 BIBLIOGRAPHY 1. Higuchi, T. (1990) Lignin biochemistry: biosynthesis and biodegradation. Wood Sci. Technol. 24: 23-63 2. Zabell, R.A. and Morell, J.J. (1992) Wood microbiology – decay and its preservation. Academic Press, Inc. New York. 3. Bu’Lock J.D. (1975) Secondary metabolism of fungi and its relation to growth and development In: The filamentous fungi, Vol. I, Ed. Smith J.E. and Berry D.R., Industrial Mycology, Wiley, NY. 4. Kirk T.K, Schultz E., Connors W.J., Lorenz L.F. and ZeikusJ.G. (1978) Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch. Microbiol. 117: 277-285 5. Keyser P., Kirk T.K. and Zeikus J.G. (1978) Ligninolytic enzyme system of Phanerochaete chrysosporium: Synthesized in the absence of lignin in response nitrogen starvation. J. Bacteriol. 135: 790-797 6. Jefferies T.W., Choi S. and Kirk T.K. (1981) Nutritional regulation of lignin degradation by Phanerochaete chrysosporium. Appl. Environ. Mirobiol. 42: 290-296 7. Kirk T.K., Connors W.J. and Zeikus J.G. (1976) Requirement for a growth substrate during lignin decomposition by two wood-rotting fungi. Appl. Environ. Microbiol. 32: 192 194 8. Reid I.D. and Seifart K.A. (1980) Lignin degradation by Phanerochaete chrysosporium in hyperbaric oxygen. Can. J. Microbiol. 26: 1168-1171 9. Yang H.H., Effland M.J. and Kirk T.K. (1980) Factors influencing fungal degradation of lignin in a representative lignocellulosic, thermomechanical pulp. Biotechnol. Bioeng. 22: 65-77 76 10. Bar-Lev S.S. and Kirk T.K. (1981) Effects of molecular oxygen on lignin degradation by Phanerochaete chrysosporium . Biochem. Biophys. Res. Commun. 99: 373-378 11. Weinstein, D.A., Krisnangkura, K., Mayfield, M.B. and Gold, M.H. (1980) Metabolism of radiolabeled β-guaiacyl ether linked lignin dimeric compounds by Phanerochaete chrysosporium. Appl. Environ. Mirobiol. 39: 535-540 12. Kirk, T.K. and Chang, H.M. (1974) Decomposition of lignin by white-rot fungi. I. Isolation of heavily degraded lignins from decayed spruce. Holzforschung 28: 217-222 13. Kirk, T.K. and Chang, H.M. (1975) Decomposition of lignin by white-rot fungi. II. Characterization of heavily degraded lignins from decayed spruce. Holzforschung 29: 56-64 14. Reid, I.D., Abrams, G.D. and Pepper, J.M. (1982) Water-soluble products from the degradation of aspen lignin by Phanerochaete chrysosporium. Can. J. Microbiol. 60: 2357-2364 15. Lundquist, K., Kirk, T.K. and Connors , W. J. (1977) Fungal degradation of Kraft lignin and liognin sulfonates prepared from synthetic 14 C- lignins. Arch. Microbiol. 112: 291-296 16. Bumpus, J. A., Tien, M., Wright, D. and Aust, S. D. (1985) Oxidation of persistent environmental pollutants by a white rot fungus. Science 228: 1434-1436. 17. Aust, S. D. (1990) Degradation of environmental pollutants by Phanerochaete chrysosporium. Microb. Ecol. 20: 197-209. 18. Vyas, B.R.M., Bakowski, S., Sasek, V., & Matucha, M. (1994) Degradation of anthracene by selected white rot fungi. FEMS Microbiol. Ecol. 14:65-70. 77 19. Stahl, J.D. and Aust, S.D. (1993) Metabolism and detoxification of TNT by Phanerochaete chrysosporium. Biochem. Biphys. Res. Commun. 192:477-482 20. Van Aken B, Cameron MD, Stahl JD, Plumat A, Naveau H, Aust SD, Agathos SN. (2000) Glutathione-mediated mineralization of 14Clabeled peroxidase 2-amino-4,6-dinitrotoluene H5 from the by white-rot manganese-dependent fungus Phanerochaete chrysosporium. Appl Microbiol Biotechnol. 54(5): 659-64. 21. Vyas, B.R.M., Sasek, V., Matucha, M and Bubner, M. (1994) Degradation of 3,3’,4,4’-tetrachlorobiphenyl by selected white rot fungi. Chemosphere 28:1127-1134. 22. Banat, I.M., Nigam, P., Singh, D., Marchant, R. (1996) Microbial decolorisation of textile-dye-containing effluents: a review. Bioresource Technol. 58: 217-227. 23. Christian, V.V., Shrivastava, R., Novotny, C., Vyas, B.R.M. (2003) Decolorization of sulfonphthalein dyes by manganese peroxidase activity of the white rot fungus Phanerochaete chrysosporium. Folia Microbiol. 48: 771-774 24. McMullan, G., Meehan, C., Conneely, A., Kirby, N., Robinson, T., Nigam, P., Banat, I. M., Marchant, R., and Smyth, W. F. (2001) Microbial decolorization and degradation of textile dyes. Appl Microbiol Biotechol 56: 81-87 25. Shrivastava, R., Christian, V., Vyas, B.R.M. Enzymatic decolorization of sulfonphthalein dyes. Enz. Microb. Technol. 36: 333-337 26. Vyas, B.R.M., and Molitoris H.P. (1995) Involvement of an extracellular H2O 2-dependent ligninolytic activity of the white rot fungus Pleurotus ostreatus in the decolorization of Remazol Brilliant blue R. Appl. Environ. Microbiol. 61: 3919-3927 78 27. Ellwardt, P.C., Haider, K. and Ernst, L. (1981) Untersuchungen des mikrobiellen spezifisch Ligninabbaues durch 13 C-angereichertem 13 C-NMR-Spektroskopie DHP-Lignin aus an Coniferylalkohol. Folzforschung 35: 103-109 28. Eriksson, K.E., Johnsrud, S.C. and Vallander, L. (1983) Degradation of lignin and lignin model compounds by various mutants of the white-rot fungus Sporotrichum pulverulentum. Arch. Microbiol. 135: 161-168 29. Glenn, J.K., and Gold, M.H. (1985) Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin degrading fungi Phanerochaete chrysosporium. Arch. Biochem . Biophys . 242: 329-341 30. Paszczynski, A., Huynh, V.B., and Crawford R. 1985. Enzymatic activities of an extracellular, manganese-dependent peroxidase from Phanerochaete chrysosporium. FEMS Microbiol. Lett. 29: 37-41 31. Kuwahara, M., Glenn, J. K., Morgan, M. A. and Gold, M. H. (1984) Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett. 169: 247-250. 32. Wariishi, H., Akileswaran, L., and Gold, M.H. (1988) Manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: spectral characterization of oxidized states and the catalytic cycle. Biochemistry 27: 5365-5370. 33. Hilden, L., Johansson, G., Pettersson, L.J., Ljungquist, P., and Henriksson, G. (2000) Do the extracellular enzyme cellbiose dehyrogenase and manganese peroxidase form a pathway in lignin biodegradation? FEBS Lett 477: 79-83 34. Tien, M. and Kirk, T. K. (1983) Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium burds. Science 221: 661-663. 79 35. Glenn, J.K., Morgan, M.A., Mayfield, M.B., Kuwahara, M. and Gold, M.H. (1983) An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem. Biophys. Res. Commun. 114: 1077-1083. 36. Tien, M. (1987) Properties of ligninase from Phanerochaete chrysosporium and their possible applications. CRC Crit. Rev. Microbiol. 15: 141-168. 37. Kirk, T. K. and Farrell, R. L. (1987) Enzymatic "combustion": The microbial degradation of lignin. Ann. Rev. Microbiol. 41: 465-505. 38. Wariishi, H. and Gold, M. H. (1989) Lignin peroxidase compound IIIformation, inactivation and conversion to the native enzyme. FEBS Lett. 243: 165-168. 39. Barr, D.P., Shah, M.M., Grover, T.A., and Aust, S.D. (1992) Production of hydroxyl radical by lignin peroxidase from Phanerochaete chrysosporium. Arch. Biochem. Biophys . 298: 480-485 40. Heinfling, A., Martinez, M.J., Martinez, A.T., Bergbauer, M. and Szewzyk, U. (1998) Purification and charac terization of peroxidase from dye-decolorizing fungus Bjerkandera adusta . FEBS Lett. 428: 141-146 41. Ruiz-Duenas , F.J., Martinez, M.J. and Martinez, A.T. (1999) Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryngii. Mol. Microbiol. 31(10): 4705-4707 42. Camarero, S., Sarkar, S., Ruiz-Duenas , F.J., Martinez, M.J. and Martinez , A.T. (1999) Description of a versatile peroxidase involved in natural degradation of lignin that has both manganese peroxidase and lignin peroxidase substrate interaction sites. J. Biol. Chem. 274(15): 10324-10330 80 43. Skorobogatko, S., Vartanov, S. and Varfolomeyev, S. D. (1994) Laccase properties, catalytic mechanism, and applicability. Appl. Biochem. Biotechnol. 49: 257–280 44. Thurston, C.F. (1994) The structure and function of fungal laccase. Microbiology 140: 19-26 45. Leontievsky, A., Myasoedova N., Pozdnykova, N. and Golovleva , L. (1997) “Yellow” laccase of Panus tigrinus oxidizes non-phenolic substrates without electron-transfer mediators. FEBS Lett. 413: 446448 46. Bourbonnais , R. and Paice, M.G. (1992) Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2,2’-azinobis-(3-ethylbenzothiozoline-6-sulphate). Appl. Microbiol. Biotechnol. 36: 823-827. 47. Farmer V.C., Henderson M.E.K. and Russel, J.D. (1960) Aromaticalcohol-oxidase activity in the growth medium of Polystictus versicolor. Biochem. J. 74: 257-262 48. Guillen, F. and Evans, C.S. (1994) Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl. Environ. Microbiol. 60: 2811-2817 49. Hammel, K.E., Mozuch, M.D., Jensen, K.A., and Kersten, P.J. (1994) H2O 2 recycling during oxidation of the arylglycerol ß-aryl ether lignin structure by lignin peroxidase and glyoxal oxidase. Biochemistry 33: 13349-13354 50. Daniel, G., Volc , J. and Kubatova , E. (1992) Pyranose oxidase, a major source of H2O2 during wood degradation by Phanerochaete chrysosporium, Trametes versicolor, and Oudemansiella mucida. Appl. Environ. Microbiol. 58: 3667-3676 81 51. Freimund, S., Huwig, A., Giffhorn , F., and Kopper, S. (1998) Rare ketoaldoses from enzymatic oxidation: substrates and oxidation products of pyranose 2-oxiase. Chem. Eur. J. 4 : 2442-2455 52. Kelley R.L., and Reddy C.A. (1986) Purification and characterization of glucose oxidase from ligninolytic cultures of Phanerochaete chrysosporium. Arch. Microbiol. 144: 254-257 53. Leonowicz , A., Rogalski, J., Jaszek, M., Luterek, J., Wojtas- Wasilewska, M., Malarczyk, E., Ginalska, G., Fink-Boots, M., and Cao N.S. (1999) Cooperation of fungal laccase and glucose 1-oxidase in transformation of Bjorkman lignin and some phenolic compounds. Holzforschung 25: 1 -20 54. Ayers, A., Ayers , S., and Eriksson, K.E. (1978) Cellobiose oxidase, purification and partial characterization of a hemoprotein from Sporotrichum pulverulentum. Eur. J. Biochem. 90: 171-181 55. Morpeth, F. (1991) Cellobiose dehydrogenase. In: Chemistry and biochemistry of flavoenzymes. Ed. Mullar, F., CRC Press, Boca Raton, Fla., pp. 337-348 56. Ander, P., Sena-Martin, G., and Duarte, J.C. (1993) Influence of cellobiose oxidase on peroxidase from Phanerochaete chrysosporium. Biochem. J. 293: 431-435 57. Volc, J., Kubatova, E., Daniel, G., and Prikrylova, V. (1996) Only C-2 specific glucose oxidase activity is expressed in ligninolytic cultures of the white rot fungus Phanerochaete chrysosporium. Arch. Microbiol. 165: 421-424 58. Harvey, P.J., Schoemaker, H. E., and Palmer, J.M. (1986) Veratryl alcohol as a media tor and the role of radical cations in lignin biodegradation by Phanerochaete chrysosporium . FEBS Lett. 195: 242-246 82 59. Hammel, K.E., Jensen, K., Mozuch, M., Landucci, L., Tien, M., and Pease, E. (1993) Ligninolysis by a purified lignin peroxidase. J. Biol. Chem. 268: 12274-12281 60. Glenn, J.K., Akileswaran, L. and Gold, M. H. (1986) Mn(II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium . Arch. Biochem. Biophys. 251: 688– 696. 61. Fenton, H.J.H. (1894) Oxidation of tartaric acid in presence of iron. J. Chem. Soc. 65: 899-910 62. Guillen, F., Munoz, C., Gomez-Toribio, V., Martinez A.T., and Martinez, M.J. (2000) Oxygen activation during oxidation of methoxyhydroquinones by laccase from Pleurotus eryngii. Appl. Enviro n. Microbiol. 66: 170–175. 63. Hadar, Y., Kerem, Z., Gorodecki, B., and Ardon, O. (1992) Utilization of lignocellulosic waste by the edible mushroom, Pleurotus. Biodegradation 3: 189-205 64. Forss, K.G., Jokinen, K., and Savolainen, M. (1977) Fermentation of wood hydrolysates. Proceeding of the TAPPI forest biology and wood chemistry conference, Atlanta. 65. Ek, M., and Eriksson, K.E. (1980) Utilization of the white-rot fungus Sporotrichum pulverulentum for water purification and protein production on mixed lignocellulosic wastewaters. Biotechnol. Bioeng. 22: 2273-2284 66. Fukuzumi, T., Nishida, A., Aoshima, K., and Minami, K. (1977) Decolorization of kraft waste liquor with white-rot fungi. I. Screening of the fungi and culturing conditions for decolorization of kraft waste liquor. Mokuzai Gakkaishi 23: 290-298 67. Crawford, D.L., and Pometto, A.L. (1984) Genetics International Inc. US Pat. 4,478,747 83 68. Cerniglia , C.E. and Sutherland, J.B. (2001) Bioremediation of polycyclic aromatic hydrocarbons by ligninolytic and non-ligninolytic fungi. In: Fungi in Bioremediation , Ed. Gadd, G.M., British Mycological Society Symposium Series, Cambridge Uni. Press. 136-187 69. Kirk, T.K. and Shimada, M. (1985) Lignin biodegradation: the microorganisms involved and the physiology and biochemistry of degradation by white-rot fungi. In: Biosynthesis and biodegradation of wood components. Ed. Higuchi, T. Academic Press, Orlando, Fl pp. 579–605. 70. Hatakka, A. Lignin-modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol. Reviews 13:125-135, 1994. 71. Lundell, T., Wever, R., Floris, R., Harvey, P., Hatakka, A., Brunow, G. and Schoemaker, H. (1993) Lignin peroxidase L3 from Phlebia radiata. Pre-steady-state and steady-state studies with veratryl alcohol and a non-phenolic lignin model compound 1-(3,4- dimethoxyphenyl)-2-(2methoxyphenoxy)propane-1,3-diol. Eur. J. Biochem. 211: 391-402, 72. Yadav, J.S., Quensen, J.F., Tiedje, J.M. and Reddy, C.A. (1995) Degradation of polychlorinated biphenyl mixtures (Aroclors 1242, 1254 and 1260) by the white rot fungus Phanerochaete chrysosporium as evidenced by congener specific analysis, Appl. Environ. Microbiol. 61: 2560-2565 73. Bumpus, J.A., Mileski, G., Brock, B., Ashbaugh, W., and Aust, S.D. (1988) Biological oxidations of organic compounds by enzymes from white rot fungus. In: Land disposal, remedial action, incineration and treatment of hazardous waste. Proceedings of the Fourteenth Annual Research Symposium. Cincinnati, OH 74. Zollinger H., (1987) Colour chemistry-synthesis, properties and applications of organic dyes and pigments. VCH, New York, pp 92-100. 84 75. O’Neill, C., Hawkes, F.R., Hawkes, D.L., Lourenco, N.D., Pinheiro, H.M. and Delee, W. (1999) Clour in textile effluents – sources, measurement, discharge consents and simulation: A Review . J. Chem. Technol. Biotechnol. 74: 1009-1018. 76. Glenn, J.K. and Gold, M.H. (1983) Decolorization of several polymeric dyes by the lignin degrading basidiomycete Phanerochaete chrysosporium, Appl. Environ. Microbiol. 45: 1741-1747. 77. Bumpus, J.A., and Brock, B.J. (1998) Biodegradation of crystal violet by the white-rot fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 54: 1140–1150. 78. Cripps , C., Bumpus , J.A. and Aust, S.D. (1990) Biodegradation of azo and heterocyclic dyes by Phaneroc haete chrysosporium. Appl. Environ. Microbiol. 159: 1114-1118. 79. Ollikka , P., Alhonmaki, K., Leppanen, V.M., Glumoff, T., Raijola, T. and Suominen, I. (1993) Decolorization of azo, triphenyl methane, heterocyclic, and polymeric dyes by lignin peroxidase isoenzymes from Phanerochaete chrysosporium . Appl. Environ. Microbiol. 59: 40104016. 80. Kling, S.H. and Neto, J.S.A. (1991) Oxidation of methylene blue by crude lignin-peroxidase from Phanerochaete chrysosporium. J. Biotechnol. 21: 295-300. 81. Conneely, A., Smyth, W.F., and McMullan G. (1999) Metabolism of the phthalocyanine textile dye Remazol turquoise blue by Phanerochaete chrysosporium. FEMS Microbiol. Lett. 179: 333-337. 82. Jarosz-Wilkolazka , A., Kochmanska-Rdest, J., Malarczyk , E., Wardas, W., and Leonowicz , A., (2002) Fungi and their ability to decolorize azo and anthraquinonic dyes . Enz. Microb. Technol. 30: 566-572. 85 83. Selvam, K., Swaminathan, K., and Chae, K.S. (2003) Decolorization of azo dyes and a dye industry effluent by a white rot fungus Thelephora sp., Biores . Technol. 88: 115-119. 84. Paszczynski, A. and Crawford, R. (1991) Degradation of azo compounds by ligninase form Phanerochaete chrysosporium: involvement of veratryl alcohol. Biochem. Biophys. Res. Commun. 178: 1056-1063. 85. Spadaro, J.T., Gold, M.H., (1992) Renganathan, V.: Degradation of azo dyes by the lignin-degrading fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 58: 2397-2401 86. Goszczynski, S., Paszczynski, A., Pasti-Grigsby, M.B., Crawford, R.L. and Crawford, D.L. (1994) New pathway for degradation of sulfonated azo dyes by microbial peroxidases of Phanerochaete chrysosporium and Streptomyces chromofuscus , J. Bacteriol. 176: 1339-1347. 87. Podgornic, H., Bradesko, L. and Perdih, A. (1995) Transformation of different dyes by extracellular ligninases from Phanerochaete chrysosporium. Biotechnol. Rev. 33: 79-83. 88. Spadaro , J.T. and Renganathan, V. (1994) Peroxidase-catalyzed oxidation of azo dyes: Mechanism of Disperse Yellow 3 degradation, Arch. Biochem. Biophys . 312: 301-307. 89. Chivukula, M. and Renganathan, V. (1995) Phenolic azo dye oxidation by laccase from Pyricularia oryzae, Appl. Environ. Microbiol. 61: 43744377. 90. Heinfling , A., Martinez, M.J., Martinez , A.J. and Bergbauer, M.S.U. (1998) Transforamtion of industrial dyes by manganese peroxidases from Bjerkandera adusta and Pleurotus eryngii in a manganeseindependent reaction. Appl. Environ. Microbiol. 64: 2788-2793. 86 91. Moreira, M.T., Palma, C., Mielo, I., Feijoo, G., and Lema, J.M. (2001) In vitro degrdation of a polymeric dye (Poly R-478) by manganese peroxidase, Biotechnol. Bioeng. 75: 362-368. 92. Schliephake, K., Mainwaring, D.E., Lonergan, G.T., Jones, I.K. and Baker, W.L. (2000) Transformation and degradation of the disazo dye Chicago Sky Blue by a purified laccase from Pycnoporus cinnabarinus. Enz. Microb. Technol. 27: 100-107. 93. Kirby, N. (1999) Bioremediation of textile industry wastewater by whiterot fungi, Ph D thesis, University of Ulster, UK. 94. Mielgo, I., Moreira, M.T., Feijoo, G., and Lema, J.M. (2002) Biodegradation of a polymeric dye in a pulsed bed bioreactor by immobilised Phanerochaete chrysosporium. Water Res. 36: 1896-1901 95. Palma, C., Moreira, M.T., Mielgo, I., Feijoo, G., and Lema, J.M. (1999) Use of a fungal bioreactor as a pretreatment or post-treatment step for continuous decolorization of dyes. Wat. Sci. Tech. 40:131–136 96. Pasti, M.B. and Crawford, D.L . (1991) Relationships between the abilities of Streptomycetes spp. to decolorize three anthron-type dyes and to degrade lignocellulose. Can. J. Microbiol. 37 : 902-907. 97. Stephenson, R.J., and Sheldon, J.B. (1996) Coagulation and precipitation of a mechanical pulping effluent. Wat. Res. 30: 781-792 98. Shah, V., Garg, N., and Madamwar, D. (1999) Exopolysaccharide production by a marine cyanobacterium Cyanothece sp., and its application in dye removal by its gelation phenomenon. Appl. Biochem. Biotechnol. 82: 81–90. 99. Haemmerli, S.D., Leisola, M.S., Sanglard, D., and Fiechter, A. (1986) Oxidation of benzo(a)pyrene by extracellular ligninases Phanerochaete chrysosporium. J. Biol. Chem. 261: 6900–6903. 87 of 100. Hammel, K., Kalyanaraman, B., and Kirk, T. (1986) Oxidation of polycyclic aromatic hydrocarbons and dibenzo[π]-dioxins by Phanerochaete chrysosporium ligninase. J. Biol. Chem. 261: 16948 – 16952. 101. Lackner, R., Srebotnik, E., and Messner, K. (1991) Oxidative degradation of high molecular weight chlorolignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem. Biophys. Acta. 178: 1092–1098. 102. Harvey, P.J., Schoemaker, H.E., and Palmer, J.M. (1985) Veratryl alcohol as a mediator and the role of radical cations in lignin biodegradation by Phanerochaete chrysosporium . FEBS Lett. 195: 242–246. 103. Haemmerli, S.D., Schoemaker, H.E., Schmidt, H.W.H., and Leisola, M.S.A. (1987) Oxidation of veratryl alcohol by the lignin peroxidase of Phanerochaete c hrysosporium. FEBS Lett. 220: 149–154. 104. Hammerich, O., and Parker, V.D. (1984) Kinetics and mechanisms of reactions of organic cation radicals in solution. Adv. Phys. Org. Chem. 20: pp. 55–189. 105. Harvey, P.J., Floris, R., Lundell, T., Palmer, J.M., Schoemaker, H.E., and Wever, R. (1992) Catalytic mechanisms and regulation of lignin peroxidase. Biochem. Soc. Trans . 20: 345–349. 106. Gierer, J., Yang, E., and Reitberger, T. (1992) The reactions of hydroxyl radicals with aromatic rings in lignins, studied with creosol and 4-methylveratrol. Holzforschung 46: 495–504. 107. Gierer, J., Yang, E., and Reitberger, T. (1994) On the significance the superoxide radical in oxidative delignification, studied with 4-tbutylsyringol and 4-t-butylguiacol. Part 1. The reaction mechanism of aromatic ring opening. Holzforschung 48: 405–414. 88 108. Shah, V. and Nerud, F. (2002) Lignin degrading system of white-rot fungi and its exploitation for dye decolorization, Can. J. Microbiol. 48: 857-870. 109. Nerud, F., Baldrian, P., Gabriel, J., and Ogbeifun, D. (2001) Decolorization of synthetic dyes by the Fenton reagent and the Cu/Pyridine/H2O2 system. Chemosphere 44: 957-961 110. Clarke, E.A., and Anliker, R. (1980) Organic dyes and pigments. In The handbook of environmental chemistry. Vol. 3. Part A. Anthropogenic compounds. Ed. Hutzinger, I., Springer-Verlag GmbH & Co. KG, Heidelberg, Germany. pp. 181–215. 111. Weber, E.J., and Wolfe, N.L. (1987) Kinetics studies of reduction of aromatic azo compounds in anaerobic sediment/water systems. Environ. Toxicol. Chem. 6: 911–920. 112. Eaton, D., Chang, H., and Kirk, T.K. (1980) Fungal decolorization of kraft bleach plant effluent. Tappi J. 63: 103–106. 113. Swamy, J., and Ramsay, J.A. (1999) The evaluation of white rot fungi in the decoloration of textile dyes. Enz. Microb. Technol. 24: 130–137. 114. Young, L., and Yu, J. (1997) Ligninase-catalyzed decolorization of synthetic dyes. Wat. Res. 31: 1187–1193. 115. Itoh, K., Kitade, Y., and Yatome, C. (1998) Oxidative biodegradation of an anthraquinone dye, pigment violet 12, by Coriolus versicolor. Bull. Environ. Contam. Toxicol. 60: 786–791 116. Yesilada, O. (1995) Decolorization of crystal violet by fungi. World J. Microbiol. Biotechnol. 11: 601-602 117. Sam, M., and Yesilada, O. (2001) Decolorization of orange II dye by white-rot fungi. Folia Microbiol. 46: 143–145. 89 118. Heinfling, A., Bergbauer, M., and Szewzyk, U. (1997) Biodegradation of azo and phthalocyanine dyes by Trametes versicolor and Bjerkandera adusta. Appl. Microbiol. Biotechnol. 48 : 261–266. 119. Heinfling, A., Martínez, M.J., Martínez, A.T., Bergbauer, M., and Szewzyk, U. (1998) Transformation of industrial dyes by manganese peroxidases from Bjerkandera adusta and Pleurotus eryngii in a manganese-independent reaction. Appl. Environ. Microbiol. 64: 2788– 2793. 120. Bhatt, M., Patel, M., Rawal, B., Novotny, C., Molitoris, H.P., and Šašek, V. (2000) Biological decolorization of synthetic dye RBBR in contaminated soil. World J. Microbiol. Biotechnol. 16: 195–198. 121. Novotny, C., Rawal, B., Bhatt, M., Patel, M., Šašek, V., and Molitoris, H.P. (2001) Capacity of Irpex lacteus and Pleurotus ostreatus for decolorization of chemically different dyes. J. Biotechnol. 89: 113–122. 122. Rodriguez, E., Pickard, M.A., and Duhalt, R.V. (1999) Industrial dye decolorization by laccases from lignolytic fungi. Curr. Microbiol. 38: 27– 32. 123. Paszczynski, A., Pasti-Grigsby, M.B., Goszczynski, S., Crawford, R.L., and Crawford, D.L. (1992) Mineralization of sulfonated azo dyes and sulfanilic acid by Phanerochaete chrysosporium and Streptomyces chromofuscus. Appl. Environ. Microbiol. 58: 598–603 124. Sayadi, S., and Ellouz, R. (1995) Roles of lignin peroxidase and manganese peroxidase from Phanerochaete chrysosporium in the decolorization of olive mill wastewaters. Appl. Environ. Microbiol. 61: 1098–1103. 125. Knapp, J.S., Newby, P.S., and Reece, L.P. (1995) Decolorization of dyes by wood-rotting basidiomycete fungi. Enz. Microb. Technol. 17: 664–668. 90 126. Freitage, M., and Morrel, J.J. (1992) Decolorization of the polymeric dye Poly R-478 by wood-inhabiting fungi. Can. J. Microbiol. 38: 811– 822. 127. Pasti-Grigsby, M.B., Paszczynski, A., Goszczynski, S., Crawford, D.L., and Crawford, R.L. (1992) Influence of aromatic substitution patterns on azo dye degradability by Streptomyces sp., and Phanerochaete chrysosporium. Appl. Environ. Microbiol. 58: 3605–3613. 128. Zilly A., Souza, C.G., Barbosa-Tessmann I.P. and Peralta R.M. (2002) Decolorization of industrial dyes by a Brazilian strain of Pleurotus pulmonarius producing laccase as the sole phenol oxidizing enzyme. Folia Microbiol. 47: 273–277. 129. Soares, G.M., Costa-Ferreira, M., and Pessoa de Amorim, M.T. (2001) Decolorization of an anthroquinone-type dye using a laccase formulation. Biores. Technol. 79: 171–177. 130. Pierce, J. 1994. Colour in textile effluents-the origins of the problem. J. Soc. Dyers Colourists 110:131-134. 131. Spadaro, J. T., I. Lorne, and V. Renganathan. 1994. Hydroxyl radical mediated degradation of azo generation. Environ. Sci. Technol. 132. dyes: evidence for benzene 28:1389-1393. Kashino, Y., T. Nishida, Y. Takahara, K. Fujita, R. Kondo, and K. Sakai. 1993. Biomechanical pulping using white rot fungus IZU-154. TAPPI. 76:167-171. 133. Fujita, K., R. Kondo, K. Sakai, Y. Kashino, T. Nishida, and Y. Takahara. 1993. Biobleaching of softwood kraft pulp with white rot fungus IZU -154. TAPPI. 76:81-84. 134. Joshi, D. K., and M. H. Gold. 1993. Degradation of 2,4,5trichlorophenol by lignin -degrading basidiomycete Phanerochaete chrysosporium. Appl. Environ. Microbiol. 59:1779-1785 91 135. Ryarden, L., and R. L. Gilbertson. 1993. European Polypores, Part I: Abortiporous - Lindtneria. (Synopsis fungorum 6) Fungiflora, Oslo, pp351-353 136. Lestan, D., and R. T. Lamar. 1996. Development of fungal inocula for bioagumentation of contaminated soils. Appl. Environ. Microbiol. 62: 2045-2052 137. Vyas B. R. M., Volc, J., and V. Sasek (1994) Ligninolytic enzymes of selected white rot fungi growing on wheat straw. Folia Microbiol. 39: 235-240 138. Ollikka, P., Alhonmaki, K., Leppanen, V.-M. Glumoff, T., Raijola, T., Suominen, I.:. Decolorization of azo, triphenyl methane, heterocyclic, and polymeric dyes by lignin peroxidase isoenzymes from Phanerochaete chrysosporium . Appl. Environ. Microbiol. 59: 40104016 (1993). 139. Field, J. A., E. de Jong, G. Feijoo Costa, and J. A. M. de Bont. 1993. Screening for ligninolytic fungi applicable to the biodegradation of xenobiotics. Trends Biotechnol. 11: 44–49 140. Swamy, J., Ramsay, J.A.: The evaluation of white rot fungi in the decolorization of textile dyes. Enzyme Microb. Technol. 24: 130-137 (1999). 141. Maximo, C., Amoriam, M.T.P., Costa-Ferreira, M.: Biotransformation of industrial reactive azo dyes by Geotrichum sp. CCMI 1019. Enz. Microb. Technol. 32, 145 -151 (2003). 142. Kirby, N., McMullan, G., Marchant, R.: Decolorization of an artificial textile effluent by Phanerochaete chrysosporium . Biotechnol. Lett. 17, 761-764 (1995). 143. Schliephake, K., Lonergan, G.T., Jones, C.L., Mainwaring, D.E.: Decolorization of pigment plant effluent by Pycnoporus cinnabarinus in packed-bed reactor. Biotechnol. Lett. 15, 1185-1188 (1993). 92 144. Shaul, G.M., Holdsworth , T.J., Demprey, C.R., and Dostal K.A. (1991) Fate of water soluble azo dyes in the activated sludge process. Chemosphere 22: 107-119 145. Morais, L.C., Freitas, O.M., Goncalves, E.P., Vasconcelos, L.T., and Gonzalez Beca, C.G. (1999) Reactive dyes removal from waste waters by adsorption on eucalyptus bark: variables that define the process. Wat. Res. 33: 979-988 146. Paszczynski, A., and Crawford , R.L. (1995) Potential of bioremediation of xenobiotic compounds by the white rot fungus Phanerochaete chrysosporium. Biotechnol. Progress. 11: 1648-1652 147. Hammel, K.E. (1992) Oxidation of aromatic pollutants by lignin degrading fungi and their extracellular peroxidases. In Metal Ions in Biological Systems, Vol. 28. Marcel Dekker, NY , 41-60 148. Nerud, F., Zouchova, Z., and Misurcova, Z. (1991) Ligninolytic properties of different white rot fungi. Biotechnol. Lett. 13: 657-660 149. Orth, A.B.D., Royse, D.J., and Tien, M. (1994) Ubiquity of lignindegrading peroxidases among various wood degrading fungi. Appl. Environ. Microbiol. 59: 4017-4023 150. Gold, M.H., Glenn, J.K., and Alic , M. (1988) Use of polymeric dyes in lignin biodegradation assays. Methods Enzymol. 161: 74-78 151. Bumpus , J.A., and Aust, S.D. (1986) Bioassays. Environ. Sci. Technol. 6: 166-170 152. Bar, D.P., and Aust, S.D. (1994) Mechanisms white rot fungi use to degrade pollutants. Environ. Sc i. Technol. 28: 79- 87 153. Cameron, M.D., Timofeevski, S., and Aust, S.D. (2000) Enzymology of Phanerochaete chrysosporium with respect to the degradation of recalcitrant compounds and xenobiotics. Appl. Microbiol. Biotechnol. 54: 751-758 93 154. Wariishi, H., Valli, K,, and Gold, M.H. (1989) Oxidative cleavage of a phenolic diarylpropane lignin model dimer by manganese peroxidase from Phanerochaete chrysosporium. Biochemistry. 28: 6017-6023. 155. Maehly, A. (1955) Plant Peroxidase. In Methods in Enzymology, Vol. II, Ed. Colowick, S., and Kaplan, N. , Academic Press, NY, 807 156. Shannon, L., Kay, E., and Lew, J.: Peroxidase Isozymes from Horseradish Roots. I. Isolation and Physical Properties, J. Biol. Chem., 241, 2166 (1966) 157. Kay, E., Shannon, L., and Lew, J. (1967) Peroxidase Isozymes from Horseradish Roots. II. Catalytic Properties, J. Biol. Chem., 242: 24702493 158. Strickland, E., Hardin, E., Shannon, L., and Horwitz, J. (1968) Peroxidase Isoenzymes from Horseradish Roots. III. Circular Dichroism of Isoenzymes and Apoisoenzymes , J. Biol. Chem. 243: 3560-3568 159. Atlas, R.M. and Unterman, R. 1999. Bioremediation. In: Industrial Microbiology & Biotechnology, 2nd Ed. , ASM Press, Washington, 666681 160. Bhunia A., Durani S., and Wangikar P.P. (2001) Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol Bioeng. 72(5): 562-567 161. Shaffiqu T.S., Roy J.J., Nair R.A., and Abraham T.E. Degradation of textile dyes mediated by plant peroxidases. Appl Biochem Biotechnol. 103(6): 315-26 162. Wesenberg D., Kyriakides I. , and Agathos S.N. (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv. 22:161-87 163. Wood, P. M. (1994) Pathways for production of Fenton’s reagent by wood-rotting fungi. FEMS Microbiol. Rev. 13: 313-320 94 164. Gallard, H., De Laat, J., and Legube, B. (2000) Comparative study of the rate of decomposition H2O2 and of atrazine by Fe(III)/ H2O 2, Cu(II)/ H2O 2. Rev. Sci. Eau.12(4): 713-728 165. Dorfman, L. M., and Adams, G. E. (1973) Reactivity of the hydroxyl radical. In national Bureau of Stardads Rep. NSRDS-NSB-46, National Bureau of Standards, Washington DC. 166. Halliwell, B., and Gutteridge, J. M. C. (1989) Free radicals in biology and medicine. Oxford University Press, Oxford. 167. Backa, S., Gierer, J., Reitberger, T., and Nilsson, T. (1993) Hydroxyl radical activity associated with the growth of brown-rot fungi. Holzforschung 47: 181-187 168. Tanaka, H., Itakura, S., and Enoki, A. (2000) Phenol oxidase activity and one-electron oxidation activity in wood degradation by soft-rot deuteromycetes. Holzforschung 54: 463-468 169. Bennet J.W., Wunch K. G., and Faison B. D. (2002) Use of fungi in biodegradation. In: Manual of Environmental Microbiology, (Ed) Hurst C.J., ASM Press, Washington DC 95 IMPLICATIONS & SPECULATIONS OF THE INVESTIGATIONS T he investigations were aimed on the potential of the lignin degrading basidiomycetes in the degradation of xenobion ts. Dyes were selected as a model of xenobionts as they represent structural diversity. Investigation was also focused on the dye decolorization by Fenton reagents, Fenton-like reagents and Horseradish peroxidase for considering an equivalent measure to evaluate the ligninolytic system of white-rot basidiomycetes (WRB). Irpex lacteus and Strain F were screened for their ability to decolorize textile dyes on solid medium. The WRB, Strain F and I. lacteus were able to decolorize the textile dyes under investigations. Variation in the decolorization of similar color dyes was observed. This is indicative that structurally similar dyes may be differentially decolorized by white rot fungi. Decolorization of blue and black dyes was followed by the change in color of lower wavelength. The degradation of dyes is not a single step reaction but follows generation of many intermediates. Dye decolorization on solid media was under older mycelia which always started from the point of inoculation and the zone of decolorization followed the zone of growth. This suggests decolorization on solid media as a part of secondary metabolic event. The correlation between the zone of growth and zone of decolorization on solid medium can be attributed to the involvement of ligninolytic activities in the decolorization. Lignin-degrading white rot fungi have received worldwide attention for their industrial use in bio-pulping, bio-bleaching, dye decolorization, and in detoxifying recalcitrant environmental pollutants. Irpex lacteus is a cosmopolitan white rot basidiomycete typically inhabiting dead hard wood trees. Decolorization of textile dyes and triphenyl methane dyes by ligninolytic 96 enzymes produced by I. lacteus during solid-state fermentation wheat straw was studied. Irpex lacteus under identical conditions produced manganese peroxidase (MnP), manganese-independent peroxidase (MIP), and lignin peroxidase (LIP). One or the other ligninolytic activities decolorized all the dyes under investigation. Manganese peroxidase was the only enzyme that was associated to the decolorization of triphenyl methane dyes. Decolorization of most of the textile dyes were attributed to MIP. Influence of pH on dye decolorization by ligninolytic activities was studied. Decolorization of textile dyes by MnP occurred at its optimal pH whereas, MIP associated decolorization varied with respect to dyes. This suggests that in defining the bioremediation program, pH must be given its due attention. The white rot fungus Pleurotus ostreatus produced manganese peroxidase (MnP) and manganese-independent peroxidase (MIP) activities during solid state fermentation of wheat straw, a natural lignocellulosic substrate. Most of the SP dyes were decolorized by MnP at pH 4.0. The higher Km for metacresol purple (40 µM) and lower Km for ortho-cresol red (26 µM) for MnP activities explained the preference for the position of methyl group at ortho than at meta on chromophore. Bromophenol blue decolorizing activity was higher at pH 3.5 and decreased as the concentration of Mn II was increased. SP-decolorizing activity was associated not only with MnP but also with MIP. Additional bromine group along with the methyl group on SP chromophores decreases the rate of decolorization. Bromination of sulfonphthalein chromophore makes them the poorer substrate for MnP. This is evident from the higher Km for bromocresol green (117 µM) when compared to bromocresol purple (36 µM) and bromophenol blue (78 µM). The order of preference for the SP dyes as substrate for the MnP- catalyzed decolorizing activity is phenol red > ortho-cresol red > meta -cresol purple > bromophenol red > bromocresol purple > bromophenol blue > bromocresol green and the order of preference for the SP dyes as substrate for the MIP - catalyzed decolorizing activity is bromocresol green > bromophenol blue > bromocresol purple > bromophenol red > meta-cresol purple > ortho-cresol red > phenol red. Inhibition of PR decolorizing activity by NaN3 provided the evidence of decolorizing activity as an oxidative process. 97 Horseradish peroxidase (HRP) is known to degrade recalcitrant organic compounds such as phenol, substituted phenols and various synthetic dyes (159). Present study focuses on the ability of HRP to degrade dyes, model of xenobionts, of varied classes (textile and triphenyl methane). We investigated the decolorization of eleven textile dyes and three triphenyl methane dyes by commercially available purified HRP. Horseradish peroxidase decolorized five of the textile dyes investigated, namely, reactive black 5 (RB5), reactive orange 16 (RO16), remazol brilliant blue R (RBBR), remazol turquoise blue G (RTBG), and remazol red H8B (RRH8B). Influence of pH on decolorization was also studied and HRP was able to decolorize dyes over broader pH range. The selection of dye as a substrate for decolorization by HRP is similar to that of manganese peroxidase of Irpex lacteus . HRP and its reaction mechanism in degradation of dyes need due attention as a candidate and in defining the strategies for the development of promising industrial waste-water treatment system. White-rot basidiomycetes (WRB) produce various is oforms of extracellular oxidases namely laccase, manganese peroxidase (MnP) and lignin peroxidase (LIP) that are involved in the degradation of lignin in the natural lignocellulosic substrates. This chapter reports the effluents decolorizing activities associated with manganese peroxidase (MnP), manganeseindependent peroxidase (MIP) and lignin peroxidase (LIP) produced by Irpex lacteus. Influence of pH and H2O2 on the ligninolytic activities was studied. Decolorization of effluents was studied by varying pH for each ligninolytic activities . MnP was the only activity that decolorized E1 better than any other ligninolytic activities investigated, whereas, in the case of E2, MnP and MIP were the activities that decolorized E2 better. the ligninolytic activities associated to decolorization of E1 showed no influence of pH and decolorized the effluent over the range of pH of investigated, whereas, efficiency of LIP to decolorize E2 decreased with the rise in pH. The differences in the decolorization of E1 and E2 can be attributed to the differences in their physico-chemical properties. 98 Fenton's reagent (FeII/H2O 2) and Fenton-like reagents (CuII/H2O2), are effective oxidants of large variety of organic pollutants. Fenton reagents are also produced from diverse routes by lignin-degrading fungi. The mechanism of decomposition of H2O 2 and of oxidation of organic solutes by Fenton's and Fenton-like reactions has been the subject of numerous studies. In our report, we present the results of our investigations of decolorization of textile, triphenyl methane dye and effluents from dyeing and printing industries by Fenton Reagent (Fe II/H2O2) and Fenton-like reagents (CuII/H2O2). Decolorization initiated immediately with the addition of hydrogen peroxide. Dyes and effluents, under investigation, were decolorized completely by Fenton reagent and Fenton-like reagent and showed hypsochromic shift. It is interesting to report that decolorization of the some of the dyes, reactive orange 16 (RO16), RRH8B, and LYF4, initiated with the increase in absorption, shifting the peak towards the higher wavelength. The reports on decolorization of dyes by white-rot fungi have shown the process of oligomerization before degradation Fenton reagent is very efficient in programming the remediatio n of textile effluents as it was able to decolorize most of the dyes completely. Redox system and mechanism of Fenton reagent needs more attention to develop it as a potent tool against the water pollution abatement programs. 99 A PPENDIX I SCHOLA RSHIPS A WARDE D Senior Research Fellowship from Department of Biotechnology (DBT), Ministry of Science and Technology, New Delhi, India (December, 2003 onwards). 100 APPENDIX II LIST OF PUBLI CATIONS AND PRESENT ATIONS PUBLICATIONS 1. Shrivastava, R., Christian, V., and Vyas, B. R. M. (2004) Enzymatic decolorization of sulfonphthalein dyes. Enz. Microb. Technol. 36: 333-337 2. Christian, V., Shrivastava, R., Shukla, D., Modi, H. A., Vyas, B.R.M. (2004) Mediator role of veratryl alcohol in the lignin peroxidasecatalyzed oxidative decolorization of Remazol Brilliant Blue R. Enz. Microb. Technol. 36: 327-332 3. Christian, V., Shrivastava, R., Shukla, D., Modi, H. A., Vyas, B.R.M. (2004) Degradation of xenobiotic compounds by lignin-degrading white-rot fungi: Enzymology and mechanisms involved. Ind. J. Exptl. Biol. (In-Press). 4. Christian, V., Shrivastava, R., Modi, H. A., Vyas, B.R.M. (2004) Physiology and biochemistry of lignin degradation by white-rot fungi. (Review article) J. Tissue Res. 3(2): 165-173 (2004). 5. Christian, V.V., Shrivastava, R., Novotny, C., Vyas, B. R. M. (2003) Decolorization of sulfonphthalein by manganese peroxidase activity of white rot fungus Phanerochaete chrysosporium. Folia Microbiol. 48(6): 771-776 101 RESEARCH ARTICLES COMMUNICATED 1. Shrivastava, R., Christian, V., Shukla, D., Modi, H.A., and Vyas, B.R.M. (2004) White-rot basidiomycetes: degradation of dyes (Review Article), Communicated to Rev. Env. Sci. Biotechnol. 2. Shrivastava, R., Christian, V., Shukla, D., Modi, H.A., and Vyas, B.R.M. (2004) Ligninolytic enzymes of Irpex lacteus : decolorization of textile dyes, triphenyl methane dyes and textile effluents, Communicated to Folia Microbiol. 3. Shrivastava, R., Christian, V., Shukla, D., Modi, H.A., and Vyas, B.R.M. (2004) Decolorization of textile dyes, triphenyl methane dyes and textile effluents by Fenton reagent and Fenton-like reagent, Communicated to Chemosphere 4. Shrivastava, R., Christian, V., Shukla, D., Modi, H.A., and Vyas, B.R.M. (2004) White-rot basidiomycetes: degradation of lignin (Review Article), Communicated to Mycologia 5. Shrivastava, R., Christian, V., Shukla, D., Modi, H.A., and Vyas, B.R.M. (2004) Horseradish peroxidase: decolorization of dyes and textile effluents. Communicated to Enz. Microb. Technol. PRESENTATIONS 1. Mehta, F., Joshi, A.P., Shrivastava, R., Christian, V.V., and Vyas, B.R.M. (2004) Decolorization of textile dyes by the wood-rotting basidiomycetes. Presented in XVIII Science Congress, March 13, Saurashtra University, R ajkot, India. 102 2. Joshi, A.P., Mehta, F., Shrivastava, R., Christian, V.V., and Vyas, B.R.M. (2004) Decolorization of textile dyes by the wood-rotting basidiomycetes. Presented in XVIII Science Congress, March 13, Saurashtra University, Rajkot, India. 3. Shrivastava, R., Christian V.V., Ladhawala K.P. and Vyas B.R.M. Influence of Mn II and NaN3 on dye decolorization capabilities of peroxidases from Pleurotus ostreatus . National Symposium on Prospecting of Fungal Diversity and Emerging Technologies & 29th Annual meeting of Mycological Society of India organized by Agharkar Research Institiute, Pune. Maharashtra. 6-7 February 2003. 4. Christian V.V., Shrivastava, R., Ladhawala K.P. and Vyas B.R.M. Decolorization of Sulphonphthalein dyes by manganese peroxidase from lignin degrading basidiomycetes Phanerochaete chrysosporium. National Symposium on Prospecting of Fungal Diversity and Emerging Technologies & 29 th Annual meeting of Mycological Society of India organized by Agharkar Research Institiute, Pune. Maharashtra. 6-7 February 2003. 5. Dave M. N., Shrivastava, R., Christian V.V., and Vyas B.R.M. Decolorization of textile dyes by the ligninolytic enzymes of white rot basidiomycetes. National Symposium on Prospecting of Fungal Diversity and Emerging Technologies & 29th Annual meeting of Mycological Society of India organized by Agharkar Research Institiute, Pune. Maharashtra. 6-7 February 2003. 6. Parekh J. T., Shrivastava, R., Christian V.V., and Vyas B.R.M. Ligninolytic enzymes of white rot basidiomycetes: Decolorization of textile dyes. National Symposium on Prospecting of Fungal Diversity and Emerging Technologies & 29th Annual meeting of Mycological Society of India organized by Agharkar Research Institiute, Pune. Maharashtra. 6 -7 February 2003. 103 7. Kesharia M. H., Christian V. V., Shrivastava, R., and Vyas B.R.M. Dye decolorization capabilities of peroxidases from Litter Basidiomycetes. National Symposium on Prospecting of Fungal Diversity and Emerging Technologies & 29th Annual meeting of Mycological Society of India organized by Agharkar Research Institiute, Pune. Maharashtra. 6-7 February 2003. 8. Shrivastava, R., Christian V.V., and Vyas B.R.M. Production profile of peroxidases and dye decolorization capabilities of white rot basidiomycetes. National Conference on Environmental Biology, Department of Biosciences, Saurashtra University, Rajkot, Gujarat. October 17-18, 2002. 9. Christian V.V., Shrivastava, R., Avlani V.A., and Vyas B.R.M. Screening of ligninolytic enzymes of selected white rot fungi in the decolorizatio n of dye. National Conference on Fungal Diversity and Biotechnology organized by Mycological Society of India, Thane, Maharashtra. February 2-4, 2002 104 A PPENDIX III CONFER ENCES / SE MINARS / WORKSHOPS ATTENDED 1. Workshop on Recombinant DNA Technology organized by Central Facility for Biotechnology Teaching and Research (CFBTR), Madurai, Tamilnadu. September 27- October 5, 2003. 2. National Symposium on Prospecting of Fungal Diversity and Emerging Technologies & 29 th Annual meeting of Mycological Society of India organized by Agharkar Research Institiute, Pune, Maharashtra. 6-7 February 2003. 3. National Science Symposium on Environmental Science and Technology organized by Commission for Scientific and Technical Terminology and Community Science Center, Rajkot, Gujarat. 6-7 January 2003. (In Hindi) 4. National Conference on Environmental Biology organized by Department of Biosciences, Saurashtra University, Rajkot, Gujarat. 1718 October 2002. 5. State level seminar on Frontiers and Advancements in Biological Science in New Millennium organized by Shree M. & N. Virani Science College, Rajkot, Gujarat. 11August 2002. 105 6. National Conference on Fungal Diversity and Biotechnology organized by Mycological Society of India, Thane , Maharashtra. 2 – 4 February 2002 7. Workshop on Principles and Methods in Regulatory Toxicology organized by Jai Research Foundation, Vapi, Gujarat. 28 – 31 May 2001. 8. National Workshop on Bioinorganic Chemistry organized by M.S. University, Baroda, Gujarat. 106