Project proposal form
advertisement

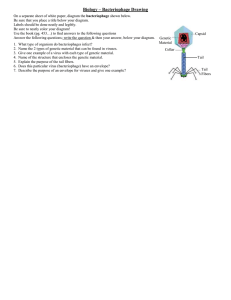
Project proposal form Project title: Marine bacteriophages: important players in antibiotic resistance gene transfer? Project code: Host institution: University of Warwick Theme: Organisms, ‘omics and biogeochemistry Key words: Antibiotic resistance, bacteriophage, marine Supervisory team (including institution & email address): Andrew Millard, University of Warwick. a.d.millard@warwick.ac.uk Dave Scanlan, University of Warwick. d.j.scanlan@warwick.ac.uk Project Highlights: Isolation of broad host range bacteriophages Functional Metagenomics to identify novel AMR genes in viral DNA Viral Metagenomics Overview: Are marine viruses important in the transfer of antimicrobial resistance (AMR) genes in the environment? The rise of antibiotic resistance is a global health problem. In the UK a report by the Chief Medical Officer, Professor Sally Davies stated that “Antimicrobial resistance poses a catastrophic threat. If we don’t act now, any one of us could go into hospital in 20 years for minor surgery and die because of an ordinary infection that can’t be treated by antibiotics. And routine operations like hip replacements or organ transplants could be deadly because of the risk of infection”. Therefore, understanding the spread and reservoir of AMR genes is of the up-most importance. To date, there has been scant attention to the marine environment as a reservoir of AMR genes, despite the Oceans covering the nd how, these genes are being transferred between bacteria is unknown. Based on the evidence found in other communities it is highly likely that bacteriophage act as mediators to transfer these AMR genes and provide a persistent reservoir of AMR genes. Recent studies on viral DNA obtained from activated sludge have demonstrated they can confer resistance to antibiotics (2), whilst in a murine model viral DNA was shown to contribute to the transfer of AMRs (3). Yet nothing is known of the role bacteriophages play in the transfer of AMR genes in the marine environment. With an estimated 1030 viruses in oceanic systems, it is essential to understand what role they may play in the dessimination of AMR genes. Given the recent finding of bacteriophage that are capable of infecting a wide range of bacterial cells from different phyla (4), bacteriophage have the potential to mediate the widespread transfer of AMR genes from the environment to pathogenic bacteria. Methodology: This PhD Project aims to answer the Figure 1: a & c : TEM image of bacteriophages isolated from seawater. B : Novel bacteriophage genome assembled from a viral metagenome. key question: What role do bacteriophage play in mediating the transfer of antibiotic resistance genes in the marine environment? The successful applicant will isolate and analyse a range of bacteriophages to determine their ability to mediate the transfer of anitbiotic resistance genes. This project is multidisciplinary in that it emcompasses both traditional microbiology, molecular biology and bioinformatics, with supervisors in both the Medical Schoool and Life Sciences. Characterisation of bacteriophages will include host range studies against a range of bacterial species; Transduction assays to determine the frequencing of horizontal gene transfer; High throughput sequencing of bacteriophage isolates; Construction of metagenomic libraries from the viral DNA fraction to identify novel AMR genes via functional genomics. Bioinformatic analysis of both bacteriophage genomes and viral metagenomes. Training and skills: CENTA students are required to complete 45 days training throughout their PhD including a 10 day placement. In the first year, students will be trained as a single cohort on environmental science, research methods and core skills. Throughout the PhD, training will progress from core skills sets to master classes specific to the student's projects and themes. More specifically, this PhD project will offer the student a unique opportunity to learn cutting edge genomics and metagenomics skills, alongside advanced bioinformatics analysis of viral metagenomes and bacteriophage genomes. Combined with the opportunity to isolate a range of novel bacteriophages against a diverse group of marine bacteria the opportunity to discover novel traits carried by these bacteriophage is clearly apparent. Partners and collaboration (including CASE): Possible timeline: Year 1: Isolation of bacteriophages infecting marine bacteria. Host range studies on bacteriophage isolates Characterisation of bacteriophages -TEM Year 2: Genome sequencing and analysis of genomes bioinformatic Year 3: Concentration of phage from seawater Construction of shotgun metagenomic libraries from phage fraction. Functional genomics to identify novel AMRs in phage fraction Confirmation of novel AMRs Characterisation of novel AMR mechanism(s) Metagenomics of viral fraction from seawater. Bioinformatic analysis of viral metagenomes Further reading: 1. Hatosy SM, Martiny AC. 2015. The ocean as a global reservoir of antibiotic resistance genes. Appl Environ Microbiol AEM.00736–15. 2. Parsley LC, Consuegra EJ, Kakirde KS, Land AM, Harper WF, Liles MR. 2010. Identification of diverse antimicrobial resistance determinants carried on bacterial, plasmid, or viral metagenomes from an activated sludge microbial assemblage. Appl Environ Microbiol 76:3753–3757. 3. Modi SR, Lee HH, Spina CS, Collins JJ. 2013. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature 499:219–22. 4. Malki K, Kula A, Bruder K, Sible E, Hatzopoulos T, Steidel S, Watkins SC, Putonti C. 2015. Bacteriophages isolated from Lake Michigan demonstrate broad host-range across several bacterial phyla. Virol J 12:164. Further details: Potential applicants are encouraged to contact: Andrew Millard (a.d.millard@warwick.ac.uk)