“Selecting the right patients for the right trials.” Paul Waring Professor of Pathology
advertisement

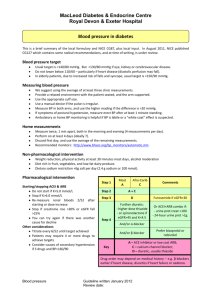
“Selecting the right patients for the right trials.” Paul Waring Professor of Pathology University of Melbourne June 21, 2011 RCPA Genetics short course Drug development challenges. Current model of drug development is becoming unsustainable Data for 1991-2000 for 10 largest pharmaceutical companies 1 in 14 drugs currently entering development is approved. Kola & Landis, Nature Reviews Drug Discovery 2004 Designer drugs Tyrosine kinase pathways in cancer R R RAS RAF SOS K K PI3-K pY pY GRB2 pY PTEN AKT MEK STAT MAPK Gene transcription Cell cycle progression P P cyclin D1 myc Cyclin D1 DNA proliferation/ maturation chemotherapy/ radiotherapy resistance Jun Fos Myc metastasis survival/anti-apoptosis angiogenesis Trastuzumab (HerceptinTM) HER2 is amplified in 15-25% of breast cancers. Disease-free survival probability 1.0 0.8 Not amplified 0.6 0.4 Amplified > 5 copies 0.2 0.0 0 12 24 36 48 Time (months) Slamon et al., Science 235:177-182, 1987 60 72 84 Herceptin’s Effects Correlate with HER2 Expression Antibody Inhibition (4D5) of Anchorage-Independent Growth HER2 Expression Level SK-BR-3 33 BT-474 25 MDA-MB-361 16.7 MDA-MB-453 16.7 MDA-MB-436 3.3 ZR-75-1 3.3 MDA-MB-231 1.2 MCF7 1.2 0 20 40 60 80 Growth Inhibition (%) 100 Her-2 amplified breast cancer Clinical trial enrichment. Negative (70%) - 35 patients ti t iidentified d tifi d ffor alternative therapy - 3% potential responders missed p - 50 unselected patients -22% benefit from therapy Positive (30%) = will benefit from therapy = will not benefit from therapy - 15 patients ti t selected l t d - 67% benefit from therapy Herceptin®: The Power of Patient Selection Unselected Target prevalence Expected survival benefit Sample size Study duration Selected (HER2+) Unselected Selected 25% 1.25m (5.7%) 11,000 349m 100% 5m (22.7%) 1,250 52m HercepTest® for Her2 screening - the first FDA - approved predictive test. Genentech approached DAKO to co-develop a commercial IHC kit. Validated during phase III by demonstrating equivalence to the clinical trial assay. Joint approval of drug and test in Sept 1998. Pathologists became the decision makers for targeted therapies. Companion Diagnostic Co-Development Imatinib (GleevecTM) Gastrointestinal stromal tumours GleevecTM and GISTs Pre Phospho-KIT Post (Day 7) Signal - seeking clinical trials Hypereosinophilia syndrome Cyr Cryptic PDFRA-FIP1LI rearrangement EGFR inhibitors EGFR is overexpressed in many common carcinomas. Overexpression of EGFR protein is rarely the result of gene amplification (except GBM). Target for EGFR inhibitorsSmall molecules (NSCLC): • Gefitinib (Iressa) • Erlotinib (Tarceva) Monoclonal antibodies (CRC): • Cetuximab (Erbitux) • Panitumumab (Vetibix) EGFR TKI in lung adenocarcinoma Lynch et al. 2004 BR.21: Tarceva monotherapy vs. placebo in chemotherapy-relapsed (2nd/3rd line) NSCLC 1.00 0.75 Survival rate TARCEVA (n=488) Placebo (n=243) Median survival (months) 6.7 4.7 1 year survival rate (%) 31.2 21.5 Overall survival: Hazard Ratio 0.73 (95% CI, 0.61-0.86)* P<0.001† 0.50 0.25 TARCEVA Placebo 0 0 6 12 18 Months 24 30 EGFR mutations Exons • Activating mutations – exon 19 deletion EGFR 18 G719X 3.2% P-loop – L858R • Overall incidence in population – ~10% in Western countries Deletion 19 48.2% Insertion 3.7% – ~30% in Asian countries DC helix Kinase domain 20 K A-loop L858R 21 42.7% Mitsudomi T, et al. Int J Clin Oncol 2006 EGFR is a founder mutation in lung adenocarcinoma. Yatabe Y. Cancer Metastasis Rev 2010 BR.21: Survival and EGFR Mutations EGFR Mutation H. EGFR Mutant EGFR Wild G. EGFR Wild Type Type 100 Placebo Log-Rank: p=0.13 HR: 0.73 (0.49,1.10) 100 60 40 20 Tarceva 60 40 20 0 At Risk Placebo Tarceva Placebo Log-Rank: p=0.45 HR: 0.77 (0.40,1.50) 80 Pe r cen t age Pe r cen t age 80 Tarceva 0 0 6 12 44 93 18 59 11 34 18 24 Time(Months) 6 9 0 1 30 0 0 Tarceva® median = 8.6 mo (n=93) Placebo median = 3.5 mo (n=44) Tsao M-S, NEJM 2005;353:133-44 At Risk Placebo Tarceva 0 6 12 19 21 10 11 5 5 18 24 Time(Months) 1 1 0 1 30 0 0 Tarceva® median = 7.4 mo (n=21) Placebo median = 7.7 mo (n=19) Sample attrition and assay failure BR.21 N % Total patients enrolled 731 100% IHC Analysis 325 44% FISH Analysis 125 17% EGFR Sequence Analysis 177 24% Patients with EGFR mutant tumors 45* 6% Total number of patients enrolled 1079 100% Total number of patients participating in optional tissue collection 710 66% Patients with samples available for analysis 479 44% Patients with samples suitable for sequencing 274 25% Patients with EGFR mutant tumors 29 3% TRIBUTE: * in 40 patients, 24 were not previously described. Eberhard DA, JCO 2005;23:5900-9, Tsao M-S, NEJM 2005;353:133-44 EGFR inhibitor prolongs survival in EGFR mutant tumours 1.0 Erlotinib (n=82) Gem/carbo (n=72) PFS probability 0.8 HR=0.16 (0.10–0.26) Log-rank p<0.0001 0.6 0.4 0.2 4.6 0 0 Patients at risk Erlotinib 82 Gem/carbo 72 5 13.1 10 15 20 Time (months) 70 51 20 2 26 4 0 0 Mechanisms of acquired resistance dodging the magic bullets. Disease Gene Drug Resistance Alternate mechanism drug NSCLC EGFR Tarceva Iressa T790M / D761Y Met Ampl XL647 / HK1272 Met SMI CML ABL Gleevec T351I +20 others Dasatinib* GIST KIT Gleevec V654A +14 others D842V Sutent* Gleevec T674I PKC412 PDGFRD HES EGFR T790M Pao W et al. PLoS Medicine 2005 PDGFRD EGFR TKI Roller-Coaster Tarceva BR21 Positive Iressa & Tarceva Ph II’s Positive Mutation is “it” Mutation does not predict survival in BR21. Ph III INTACT I/II, TALENT, TRIBUTE Negative 2000 2003 FISH is “it” PhIII IPASS Adjuvant positive study in FISH + Commercial concerns over patient selection Ph IV SATURN IHC negative Iressa ISEL Negative 2004 2005 2008 Targeted therapies The right trial The right target The right patients (biomarkers) The right drug The right disease subset (indication) Break-apart FISH assay 2p23 region t(2;5) ALK gene breakpoint region 3’ ~250 kb 5’ ~300 kb EML4-ALK frequency: ~4-10% Primary lung adenocarcinoma Soda et al., Nature 448: 561-566, 2007 Tumor Responses to PF-02341066 for Evaluable NSCLC ALK Patients Tumor Size Change and Treatment Duration (weeks) % of best change from baseline 3 10 7 10 19 30 20 16 11 16 15 59% 6 22 2 RR Overall (17/29) 17 21 16 8 41 11 26 41 46 19 40 20 0 -20 -40 3 10 7 6 22 2 16 15 20 16 -60 11 10 19 30 17 21 16 8 41 11 26 41 46 -80 -100 19 Green - PR Blue - SD Black - PD Two patients had clinical progression and discontinued without radiographic confirmation. Vemurafenib inhibits BRAFV600E Kinase RTK RAS 40-60% of melanomas BRAF RAF V600E VEMURAFENIB ATP MEK ATP ERK Cellular Proliferation (PLX4032, RO5185426) PLX4032 Progression-free survival (%) BRIM-3 Progression-free survival 100 Hazard Ratio 0.26 90 (95% CI; 0.20 - 0.33) Log-rank P<0.0001 Vemurafenib (N=275) 80 70 Dacarbazine (N=274) 60 50 40 30 Median 5.3 mos Median 1.6 mos 20 10 0 0 1 No. of patients in follow up 2 3 4 5 6 7 Months 8 9 10 11 12 BRIM -3 Overall survival 100 Vemurafenib (N=336) Est 6 mo survival 84% Overall survival (%) 90 80 70 Dacarbazine (N=336) Est 6 mo survival 64% 60 50 40 30 Hazard ratio 0.37 20 (95% CI; 0.26 - 0.55) Log-rank P<0.0001 10 0 0 1 2 3 4 5 6 7 Months 8 9 10 11 12 Cetuximab (ErbituxTM). 75% expression 10% amplified Ligand 0% mutant EGFR Cetuximab K K PI3K Grb-2 SOS RAS RAF PTEN AKT mTOR STAT3/5 Survival MEK MAPK Proliferation Ligand EGFR Cetuximab K K PI3K Grb-2 SOS RAS 35% mutant RAF PTEN AKT mTOR STAT3/5 Survival MEK MAPK Proliferation Ligand EGFR Cetuximab K K 15-25% mutant PI3K Grb-2 SOS RAS 35% mutant RAF 10% mutant 40% loss PTEN AKT mTOR STAT3/5 Survival MEK MAPK Proliferation Mutations in PTCH and SMO are found in hereditary & sporadic basal cell carcinomas Inactive receptor Loss of PTCH mutations Ligand-dependent activation Activating SMO mutations •PTCH/Gli1 •IGF •Cyclin D1 •Other targets Tumour growth A naturally occurring inhibitor of SMO showed that the Hedgehog pathway was drugable. Shepherds in Idaho in 1950s identified cyclopic lambs. Pregnant ewes feeding on corn lily plants. The teratogen, cyclopamine, targets SMO. GDC-0449 Phase 1 trial GDC-0449 in metastatic BCC Partial response with multiple daily doses (150 mg) of GDC-0449 Baseline After 8 weeks After 16 weeks 8/9 patients treated so far have had benefit. BRCA1&2 - deficient tumors are defective in homologous recombination PARP repairs DNA single strand breaks Synthetic lethality Guha M. Nature Biotechnology 2011: 29:373-374 Trade name Target Type Company TGA approved use. ( *pending) Trastuzumab (Herceptin®) HER2 McAb Genentech / Roche Met and adjuvant HER2+ breast cancer Metastatic HER2+ gastric cancer* Cetuximab (Erbitux®) EGFR McAb Imclone / BMS / Merck EGFR + KRAS wt Metastatic CRC H & N SCC Panitumumab (Vectibix®) EGFR TK SMI Amgen EGFR + KRAS wt Metastatic CRC Gefitinib (Iresssa® ) EGFR TK SMI AstraZeneca 1L EGFR mut metastatic NSCLC* Erlotinib (Tarceva®) EGFR TK SMI Genentech / OSI / Roche 2L NSCLC 1L EGFR mut metastatic NSCLC* Crizotinib ALK SMI Pfizer EML4-ALK+ NSCLC* Imatinib (Gleevec®) ABL KIT PDGFR TK SMI Novartis CML Adult Ph+ ALL GIST DFSP Hypereosinophilia syndrome PDGFR rearranged MDS/MPD Systemic mastocytosis Dasatinib (Sprycel®) ABL TK SMI BMS Gleevec resistant CML Gleevec resistant Adult Ph+ ALL Sunitinib (Sutent®) KIT VEGFR TK SMI Pfizer Gleevec resistant GIST Met renal cell cancer Vemerafenib BRAF TK SMI Plexikon / Roche BRAF V600E + metastatic melanoma* Olaparib PARP SMI AstraZeneca BRCA1 & 2 mut Breast cancer* BRCA1 & 2 mut Ovarian cancer* Pharmaceutical oncology pipeline Project Initiation Clinical Candidate Selection Research IND Filing Phase III Decision Early Development Phase I Phase II Phase III Success rate is now 1 in 5